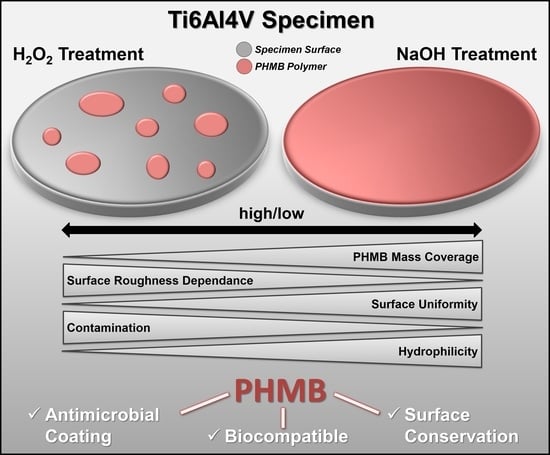
Improved Adsorption of the Antimicrobial Agent Poly (Hexamethylene) Biguanide on Ti-Al-V Alloys by NaOH Treatment and Impact of Mass Coverage and Contamination on Cytocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. In vitro Experiments
2.1.1. Ti6Al4V Test Specimens
2.1.2. Poly (Hexamethylene) Biguanide Hydrochloride and Chlorhexidine Digluconate
2.1.3. Surface Treatment Procedures
2.1.4. Quantification of Adsorbed Biguanides
2.1.5. Surface Analysis
2.1.6. Determination of Anionic Functional Groups on the Ti6Al4V Surface
2.1.7. Detection of Adsorbed Biguanides on the Ti6Al4V Surface Using Rose Bengal
2.1.8. SEM for Assessment of the Surface Structure
2.1.9. Wettability
2.1.10. Binding of FITC to PHMB and Coating of the Test Specimen
2.1.11. SaOs-2 Cell Culture, Cell Viability, Alkaline Phosphatase Activity and Mineralization Assay
2.2. Molecular Dynamics Simulations
2.2.1. PHMB Models and Force Field
2.2.2. Simulation Settings
2.2.3. Model Building and Simulated Systems
- (a)
- The amino and cyanoguanidino end-groups as opposing interfaces by assembling repeats of 6X × 7Y × 1Z periodic copies resulting in a surface of 33.5 nm × 29.6 nm × 11 nm, representing specific adsorption of end-groups to the Ti6Al4V disc and exposing the respective other end to the water droplet.
- (b)
- The main chain unit as interface by repeats of 6X × 2Y × 3Z of size 33.5 nm × 8.4 nm × 33.1 nm, representing flat multi-layered aggregation. This model is also considered representative for an amorphous aggregation, since there is no preference on parts of the polymer to be water exposed.
2.2.4. Time Dependent Contact Angle Analysis
3. Results
3.1. Theoretical Modelling
3.1.1. Isolated PHMB Oligomer
3.1.2. PHMB Micelle Model
3.1.3. Simulated Water Contact Angles
3.2. In Vitro Experiments
3.2.1. Carbon Contamination and Water Contact Angle
3.2.2. Til6Al4V Surface Treatment with NaOH or H2O2 and Resulting Biguanide Adsorption
3.2.3. Staining of Anionic Equivalents
3.2.4. Adsorption of FITC-PHMB on Test-Specimen
3.2.5. SEM Showed Differences in Surface Texture
3.2.6. Cell Viability, Activity of Alkaline Phosphatase and Calcium Apatite Formation
4. Discussion
4.1. Structure and Effect of PHMB
4.2. Simulated Wetting Properties
4.3. Effects of Surface Treatment on Adsorption
4.4. Osseointegration in the Presence and Absence of PHMB Coating
4.5. Surface Composition and Influence on PHMB Adsorption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Supporting Figures



References
- Hierro-Oliva, M.; Gallardo-Moreno, A.M.; Gonzalez-Martin, M.L. XPS Analysis of Ti6Al4V Oxidation Under UHV Conditions. Metall. Mater. Trans. A 2014, 45, 6285–6290. [Google Scholar] [CrossRef]
- Da Fonseca, C.; Boudin, S.; da Cunha Belo, M. Characterisation of titanium passivation films by in situ ac impedance measurements and XPS analysis. J. Electroanal. Chem. 1994, 379, 173–180. [Google Scholar] [CrossRef]
- Kang, B.S.; Sul, Y.T.; Oh, S.J.; Lee, H.J.; Albrektsson, T. XPS, AES and SEM analysis of recent dental implants. Acta Biomater. 2009, 5, 2222–2229. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. Part A 2005, 74A, 49–58. [Google Scholar] [CrossRef]
- Lim, Y.J.; Oshida, Y. Initial contact angle measurements on variously treated dental/medical titanium materials. Bio-Med. Mater. Eng. 2001, 11, 325–341. [Google Scholar]
- Sakai, N.; Fukuda, K.; Shibata, T.; Ebina, Y.; Takada, K.; Sasaki, T. Photoinduced Hydrophilic Conversion Properties of Titania Nanosheets. J. Phys. Chem. B 2006, 110, 6198–6203. [Google Scholar] [CrossRef]
- Dhaliwal, J.; David, S.; Zulhilmi, N.; Dhaliwal, S.; Knights, J.; Junior, R. Contamination of titanium dental implants: A narrative review. SN Appl. Sci. 2020, 2, 1011. [Google Scholar] [CrossRef]
- Müller, G.; Benkhai, H.; Matthes, R.; Finke, B.; Friedrichs, W.; Geist, N.; Langel, W.; Kramer, A. Poly (hexamethylene biguanide) adsorption on hydrogen peroxide treated Ti–Al–V alloys and effects on wettability, antimicrobial efficacy, and cytotoxicity. Biomaterials 2014, 35, 5261–5277. [Google Scholar] [CrossRef]
- Hornschuh, M.; Zwicker, P.; Schmidt, T.; Finke, B.; Kramer, A.; Müller, G. Poly (hexamethylene biguanide), adsorbed onto Ti-Al-V alloys, kills slime-producing Staphylococci and Pseudomonas aeruginosa without inhibiting SaOs-2 cell differentiation. J. Biomed. Mater. Res. Part Appl. Biomater. 2019, 108, 1801–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groll, J.; Fiedler, J.; Bruellhoff, K.; Moeller, M.; Brenner, R.E. Novel Surface Coatings Modulating Eukaryotic Cell Adhesion and Preventing Implant Infection. Int. J. Artif. Organs 2009, 32, 655–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, A.; Eberlein, T.; Müller, G.; Dissemond, J.; Assadian, O. Re-evaluation of polihexanide use in wound antisepsis in order to clarify ambiguities of two animal studies. J. Wound Care 2019, 28, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Hornschuh, M.; Zwicker, P.; Schmidt, T.; Kramer, A.; Müller, G. In vitro evaluation of contact-active antibacterial efficacy of Ti-Al-V alloys coated with the antimicrobial agent PHMB. Acta Biomater. 2020, 106, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Tengvall, P.; Elwing, H.; Sjöqvist, L.; Lundström, I.; Bjursten, L. Interaction between hydrogen peroxide and titanium: A possible role in the biocompatibility of titanium. Biomaterials 1989, 10, 118–120. [Google Scholar] [CrossRef]
- Köppen, S.; Langel, W. Simulation of the interface of (100) rutile with aqueous ionic solution. Surf. Sci. 2006, 600, 2040–2050. [Google Scholar] [CrossRef]
- Friedrichs, W.; Langel, W. Atomistic modeling of peptide adsorption on rutile (100) in the presence of water and of contamination by low molecular weight alcohols. Biointerphases 2014, 9, 031006. [Google Scholar] [CrossRef]
- O’Malley, L.P.; Hassan, K.Z.; Brittan, H.; Johnson, N.; Collins, A.N. Characterization of the biocide polyhexamethylene biguanide by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Appl. Polym. Sci. 2006, 102, 4928–4936. [Google Scholar] [CrossRef]
- Bratt, H.; Hathway, D.E. Characterization of the urinary polymer-related material from rats given poly[biguanide-1,5-diylhexamethylene hydrochloride]. Die Makromol. Chem. 1976, 177, 2591–2605. [Google Scholar] [CrossRef]
- Sano, S.; Kato, K.; Ikada, Y. Introduction of functional groups onto the surface of polyethylene for protein immobilization. Biomaterials 1993, 14, 817–822. [Google Scholar] [CrossRef]
- Gilbert, P.; Pemberton, D.; Wilkinson, D.E. Synergism within polyhexamethylene biguanide biocide formulations. J. Appl. Bacteriol. 1990, 69, 593–598. [Google Scholar] [CrossRef]
- Gilbert, P.; Pemberton, D.; Wilkinson, D.E. Barrier properties of the Gram-negative cell envelope towards high molecular weight polyhexamethylene biguanides. J. Appl. Bacteriol. 69 1990, 69, 585–592. [Google Scholar] [CrossRef]
- Chindera, K.; Mahato, M.; Sharma, A.K.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.; Kumar, S.; Mcfarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, G.; Vlieg, E.; Vriend, G. Molden 2.0: Quantum chemistry meets proteins. J. Comput. Aided Mol. Des. 2017, 31, 789–800. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Case, D.; Ben-Shalom, I.; Brozell, S.; Cerutti, D.; Cheatham, T., III; Cruzeiro, V.; Darden, T.; Duke, R.; Ghoreishi, D.; Gilson, M.; et al. AMBER 2018; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
- Case, D.A.; Darden, T.A.; Cheatham, T.E., III; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Crowley, M.; Walker, R.C.; Zhang, W.; et al. AMBER 10; University of California: San Francisco, CA, USA, 2008. [Google Scholar]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef]
- NVIDIA; Vingelmann, P.; Fitzek, F.H. CUDA. Release: 10.2.89. 2020. [Google Scholar]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, C.W.; Grand, S.L.; Walker, R.C.; Roitberg, A.E. Long-Time-Step Molecular Dynamics through Hydrogen Mass Repartitioning. J. Chem. Theory Comput. 2015, 11, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.C. Rattle: A “velocity” version of the shake algorithm for molecular dynamics calculations. J. Comput. Phys. 1983, 52, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Grest, G.S.; Kremer, K. Molecular dynamics simulation for polymers in the presence of a heat bath. Phys. Rev. A 1986, 33, 3628. [Google Scholar] [CrossRef]
- Feller, S.E.; Zhang, Y.; Pastor, R.W.; Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD—Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- De Paula, G.F.; Netto, G.I.; Mattoso, L.H.C. Physical and Chemical Characterization of Poly(hexamethylene biguanide) Hydrochloride. Polymers 2011, 3, 928–941. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C. Gnuplot 5.0.6: An Interactive Plotting Program. 2017. Available online: http://gnuplot.sourceforge.net/ (accessed on 5 August 2021).
- McClellan, A.; Harnsberger, H. Cross-sectional areas of molecules adsorbed on solid surfaces. J. Colloid Interface Sci. 1967, 23, 577–599. [Google Scholar] [CrossRef]
- Ikeda, T.; Tazuke, S.; Watanabe, M. Interaction of biologically active molecules with phospholipid membranes: I. Fluorescence depolarization studies on the effect of polymeric biocide bearing biguanide groups in the main chain. Biochim. Biophys. Acta (BBA) Biomembr. 1983, 735, 380–386. [Google Scholar] [CrossRef]
- Ikeda, T.; Ledwith, A.; Bamford, C.; Hann, R. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim. Biophys. Acta (BBA) Biomembr. 1984, 769, 57–66. [Google Scholar] [CrossRef]
- Zaki, A.M.; Troisi, A.; Carbone, P. Unexpected Like-Charge Self-Assembly of a Biguanide-Based Antimicrobial Polyelectrolyte. J. Phys. Chem. Lett. 2016, 7, 3730–3735. [Google Scholar] [CrossRef]
- Lin, J.; Alexander-Katz, A. Cell Membranes Open “Doors” for Cationic Nanoparticles/Biomolecules: Insights into Uptake Kinetics. ACS Nano 2013, 7, 10799–10808. [Google Scholar] [CrossRef]
- Sowlati-Hashjin, S.; Carbone, P.; Karttunen, M. Insights into the Polyhexamethylene Biguanide (PHMB) Mechanism of Action on Bacterial Membrane and DNA: A Molecular Dynamics Study. J. Phys. Chem. B 2020, 124, 4487–4497. [Google Scholar] [CrossRef]
- Fauquignon, M.; Ibarboure, E.; Carlotti, S.; Brûlet, A.; Schmutz, M.; Meins, J.F.L. Large and Giant Unilamellar Vesicle(s) Obtained by Self-Assembly of Poly(dimethylsiloxane)-b-poly(ethylene oxide) Diblock Copolymers, Membrane Properties and Preliminary Investigation of Their Ability to Form Hybrid Polymer/Lipid Vesicles. Polymers 2019, 11, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creppy, E.; Aboudoulatif, D.; Serge, M.; Eklu-Gadegbeku, C.; Cros, D. Study of Epigenetic Properties of Poly(HexaMethylene Biguanide) Hydrochloride (PHMB). Int. J. Environ. Res. Public Health 2014, 11, 8069–8092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, E.; Schulze, R.; Liu, C.; Dombrowski, D. Influence of surface contamination on the wettability of heat transfer surfaces. Int. J. Heat Mass Transf. 2015, 91, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.; Denzer, A.; Cochran, D.; Hoffmann, B.; Lussi, A.; Steinemann, S. Enhanced Bone Apposition to a Chemically Modified SLA Titanium Surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, D.; Deo, N.; Markovic, B.; Stranick, M.; Somasundaran, P. Adsorption and dissolution behavior of human plasma fibronectin on thermally and chemically modified titanium dioxide particles. Biomaterials 2002, 23, 1269–1279. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zwicker, P.; Geist, N.; Göbler, E.; Kulke, M.; Schmidt, T.; Hornschuh, M.; Lembke, U.; Prinz, C.; Delcea, M.; Kramer, A.; et al. Improved Adsorption of the Antimicrobial Agent Poly (Hexamethylene) Biguanide on Ti-Al-V Alloys by NaOH Treatment and Impact of Mass Coverage and Contamination on Cytocompatibility. Coatings 2021, 11, 1118. https://doi.org/10.3390/coatings11091118
Zwicker P, Geist N, Göbler E, Kulke M, Schmidt T, Hornschuh M, Lembke U, Prinz C, Delcea M, Kramer A, et al. Improved Adsorption of the Antimicrobial Agent Poly (Hexamethylene) Biguanide on Ti-Al-V Alloys by NaOH Treatment and Impact of Mass Coverage and Contamination on Cytocompatibility. Coatings. 2021; 11(9):1118. https://doi.org/10.3390/coatings11091118
Chicago/Turabian StyleZwicker, Paula, Norman Geist, Elisabeth Göbler, Martin Kulke, Thomas Schmidt, Melanie Hornschuh, Ulrich Lembke, Cornelia Prinz, Mihaela Delcea, Axel Kramer, and et al. 2021. "Improved Adsorption of the Antimicrobial Agent Poly (Hexamethylene) Biguanide on Ti-Al-V Alloys by NaOH Treatment and Impact of Mass Coverage and Contamination on Cytocompatibility" Coatings 11, no. 9: 1118. https://doi.org/10.3390/coatings11091118