Effectiveness and Compatibility of Nanoparticle Based Multifunctional Coatings on Natural and Man-Made Stones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Formulation
2.2. Stone Samples
2.3. Coating Deposition
2.4. Characterization Techniques
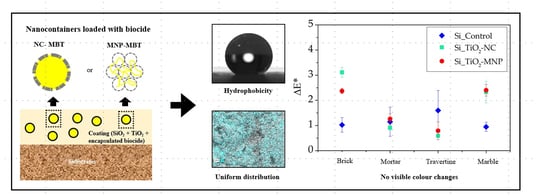
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vidal, F.; Vicente, R.; Mendes Silva, J. Review of environmental and air pollution impacts on built heritage: 10 questions on corrosion and soiling effects for urban intervention. J. Cult. Herit. 2019, 37, 273–295. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.; Benavente, D.; Gomez-Heras, M.; Marco-Castaño, L.; García-Del-Cura, M.Á. Non-linear decay of building stones during freeze-thaw weathering processes. Constr. Build. Mater. 2013, 38, 443–454. [Google Scholar] [CrossRef]
- Cardell, C.; Delalieux, F.; Roumpopoulos, K.; Moropoulou, A.; Auger, F.; Van Grieken, R. Salt-induced decay in calcareous stone monuments and buildings in a marine environment in SW France. Constr. Build. Mater. 2003, 17, 165–179. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef]
- Young, M.E.; Alakomi, H.L.; Fortune, I.; Gorbushina, A.A.; Krumbein, W.E.; Maxwell, I.; McCullagh, C.; Robertson, P.; Saarela, M.; Valero, J.; et al. Development of a biocidal treatment regime to inhibit biological growths on cultural heritage: BIODAM. Environ. Geol. 2008, 56, 631–641. [Google Scholar] [CrossRef]
- Arai, T.; Harino, H.; Ohji, M. Ecotoxicology of Antifouling Biocides; Langston, W.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Srinivasan, M.; Swain, G.W. Managing the use of copper-based antifouling paints. Environ. Manag. 2007, 39, 423–441. [Google Scholar] [CrossRef]
- Imbesi, P.M.; Gohad, N.V.; Eller, M.J.; Orihuela, B.; Rittschof, D.; Schweikert, E.A.; Mount, A.S.; Wooley, K.L. Noradrenaline-functionalized hyperbranched fluoropolymer–poly(ethylene glycol) cross-linked networks as dual-mode, anti-biofouling coatings. ACS Nano 2012, 6, 1503–1512. [Google Scholar] [CrossRef]
- Jämsä, S.; Mahlberg, R.; Holopainen, U.; Ropponen, J.; Savolainen, A.; Ritschkoff, A.C. Slow release of a biocidal agent from polymeric microcapsules for preventing biodeterioration. Prog. Org. Coat. 2013, 76, 269–276. [Google Scholar] [CrossRef]
- Ruggiero, L.; Di Bartolomeo, E.; Gasperi, T.; Luisetto, I.; Talone, A.; Zurlo, F.; Peddis, D.; Ricci, M.A.; Sodo, A. Silica nanosystems for active antifouling protection: Nanocapsules and mesoporous nanoparticles in controlled release applications. J. Alloys Compd. 2019, 798, 144–148. [Google Scholar] [CrossRef]
- Mardones, L.E.; Legnoverde, M.S.; Monzón, J.D.; Bellotti, N.; Basaldella, E.I. Increasing the effectiveness of a liquid biocide component used in antifungal waterborne paints by its encapsulation in mesoporous silicas. Prog. Org. Coat. 2019, 134, 145–152. [Google Scholar] [CrossRef]
- Dresler, C.; Saladino, M.L.; Demirbag, C.; Caponetti, E.; Chillura Martino, D.F.; Alduina, R. Development of controlled release systems of biocides for the conservation of cultural heritage. Int. Biodeterior. Biodegrad. 2017, 125, 150–156. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, R.; Su, M.; Li, H.; Yue, X.; Qin, D.; Jiang, Z. Functionalization of halloysite nanotubes by enlargement and layer-by-layer assembly for controlled release of the fungicide iodopropynyl butylcarbamate. RSC Adv. 2019, 9, 42062–42070. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, L.; Bartoli, F.; Fidanza, M.R.; Zurlo, F.; Marconi, E.; Gasperi, T.; Tuti, S.; Crociani, L.; Di Bartolomeo, E.; Caneva, G.; et al. Encapsulation of environmentally-friendly biocides in silica nanosystems for multifunctional coatings. Appl. Surf. Sci. 2020, 514, 145908. [Google Scholar] [CrossRef]
- Salazar-Hernández, C.; Zárraga, R.; Alonso, S.; Sugita, S.; Calixto, S.; Cervantes, J. Effect of solvent type on polycondensation of TEOS catalyzed by DBTL as used for stone consolidation. J. Sol-Gel Sci. Technol. 2009, 49, 301–310. [Google Scholar] [CrossRef]
- Mosquera, M.J.; Bejarano, M.; De la Rosa-Fox, N.; Esquivias, L. Producing crack-free colloid-polymer hybrid gels by tailoring porosity. Langmuir 2003, 19, 951–957. [Google Scholar] [CrossRef]
- Angulo-Olais, R.; Illescas, J.F.; Aguilar-Pliego, J.; Vargas, C.A.; Haro-Pérez, C. Gel point determination of TEOS-based polymeric materials with application on conservation of cultural heritage buildings. Adv. Condens. Matter Phys. 2018, 2018, 5784352. [Google Scholar] [CrossRef] [Green Version]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2-SiO2-PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Pinho, L.; Elhaddad, F.; Facio, D.S.; Mosquera, M.J. A novel TiO2-SiO2 nanocomposite converts a very friable stone into a self-cleaning building material. Appl. Surf. Sci. 2013, 275, 389–396. [Google Scholar] [CrossRef]
- Pinho, L.; Mosquera, M.J. Photocatalytic activity of TiO2-SiO2 nanocomposites applied to buildings: Influence of particle size and loading. Appl. Catal. B Environ. 2013, 134–135, 205–221. [Google Scholar] [CrossRef]
- Ruggiero, L.; Fidanza, M.R.; Iorio, M.; Tortora, L.; Caneva, G.; Ricci, M.A.; Sodo, A. Synthesis and characterization of TEOS coating added with innovative antifouling silica nanocontainers and TiO2 nanoparticles. Frontiers (Boulder) 2020, 7, 1–11. [Google Scholar] [CrossRef]
- Xu, F.; Li, D.; Zhang, Q.; Zhang, H.; Xu, J. Effects of addition of colloidal silica particles on TEOS-based stone protection using n-octylamine as a catalyst. Prog. Org. Coat. 2012, 75, 429–434. [Google Scholar] [CrossRef]
- Haake, S.; Simon, S.; Favaro, M. The bologna cocktail-evaluation of consolidation treatments on monuments in france and italy after 20 years on natural aging. In Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, Stockholm, Sweden, 27 June–2 July 2004; pp. 423–430. [Google Scholar]
- UNI EN 15801:2010 Conservation of Cultural Property—Test Methods—Determination of Water Absorption by Capillarity; Ente Nazionale Italiano di Unificazione (UNI): Milan, Italy, 2010.
- DIN 52 615 Testing of Thermal Insulating Material: Determination of Water Vapour Permeability of Construction and Insulating Materials; Deutsches Institut fur Normung E.V. (DIN): Berlin, Germany, 1987.
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81A, 89. [Google Scholar] [CrossRef]
- Normal 43/93 Misure Colorimetriche di Superfici Opache (Italian Normative on Stone Material-Colorimetric Measurement of Opaque Surfaces); Commissione Beni Culturali Uni Normal: Roma, Italy, 1993.
- Delgado Rodrigues, J.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Sasse, H.S.; Snethlage, R. Methods for the evaluation of stone conservation treatments. In Report of Dahlem Workshop on Saving our Architectural Heritage, Berlin; Baer, N.S., Snethlage, R., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 1996; pp. 223–243. [Google Scholar]
- Hobson, T. Parameters and Definitions for Roundness and Surface Measurements; Taylor Hobson Ltd.: Leicester, UK, 2000. [Google Scholar]
- Cappelletti, G.; Fermo, P. Hydrophobic and Superhydrophobic Coatings for Limestone and Marble Conservation; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9781782422839. [Google Scholar]
- Esmeryan, K.D. Critical aspects in fabricating multifunctional super-nonwettable coatings exhibiting icephobic and anti-biofouling properties. Coatings 2021, 11, 339. [Google Scholar] [CrossRef]





| Samples | Morphology | Diameter (nm) | Loading Capability (Weight %) | Surface Area (m2/g) |
|---|---|---|---|---|
| NC | Spherical | 140 ± 20 | 10 | - |
| MNP | Spherical | 39 ± 4 | 8.2 | - |
| Si-Control | - | - | - | 440 |
| Si-TiO2-NC | - | - | - | 489 |
| Si-TiO2-MNP | - | - | - | 488 |
| Sample | Average Static Contact Angle (°) | ||
|---|---|---|---|
| Si_Control | Si_TiO2-NC | Si_TiO2-MNP | |
| Brick | 125.7 ± 4.7 | 143.4 ± 5.2 | 140.1 ± 5.5 |
| Mortar | 127.7 ± 3.5 | 140.1 ± 2.2 | 127.2 ± 3.9 |
| Travertine | 118.9 ± 5.1 | 119.7 ± 3.2 | 107.5 ± 5.5 |
| Carrara Marble | 125.5 ± 4.2 | 135.6 ± 2.9 | 128.6 ± 3.4 |
| Sample | Reduction in CWAC (%) | ||
|---|---|---|---|
| Si_Control | Si_TiO2-NC | Si_TiO2-MNP | |
| Brick | 96.49 | 99.03 | 98.46 |
| Mortar | 98.97 | 99.28 | 99.46 |
| Travertine | 10.52 | 39.87 | 53.70 |
| Carrara marble | 46.25 | 57.47 | 86.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuena, M.; Ruggiero, L.; Della Ventura, G.; Bemporad, E.; Ricci, M.A.; Sodo, A. Effectiveness and Compatibility of Nanoparticle Based Multifunctional Coatings on Natural and Man-Made Stones. Coatings 2021, 11, 480. https://doi.org/10.3390/coatings11040480
Zuena M, Ruggiero L, Della Ventura G, Bemporad E, Ricci MA, Sodo A. Effectiveness and Compatibility of Nanoparticle Based Multifunctional Coatings on Natural and Man-Made Stones. Coatings. 2021; 11(4):480. https://doi.org/10.3390/coatings11040480
Chicago/Turabian StyleZuena, Martina, Ludovica Ruggiero, Giancarlo Della Ventura, Edoardo Bemporad, Maria Antonietta Ricci, and Armida Sodo. 2021. "Effectiveness and Compatibility of Nanoparticle Based Multifunctional Coatings on Natural and Man-Made Stones" Coatings 11, no. 4: 480. https://doi.org/10.3390/coatings11040480