Contribution of Different Pretreatments to the Thermal Stability and UV Resistance Performance of Cellulose Nanofiber Films
Abstract
:1. Introduction
2. Experiments
2.1. Materials
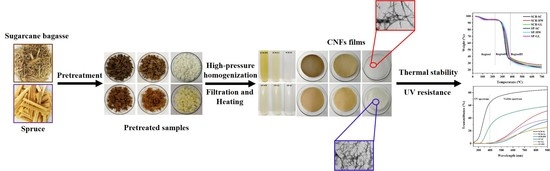
2.2. Pretreatments
2.3. Preparation of Cellulose Nanofibers
2.4. Chemical Composition Analysis of Cellulose Nanofibers
2.5. Characterization of Cellulose Nanofibers
2.6. Thermogravimetry (TGA) Analysis
2.7. UV Absorption Performance
3. Results and Discussion
3.1. Chemical Composition of CNFs
3.2. Photography and TEM Analysis
3.3. FTIR Analysis
3.4. XPS Analysis
3.5. X-ray Diffraction (XRD) Analysis
3.6. Thermogravimetry (TGA) Analysis
3.7. Analysis of UV Absorption Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fu, H.; Liu, H.; Yu, K.; Wang, Y.; Ma, J. In-situ packaging ultra-uniform 3D hematite nanotubes by polyaniline and their improved gas sensing properties. Mater. Res. Bull. 2018, 107, 46–53. [Google Scholar] [CrossRef]
- Farooq, A.; Jiang, S.; Farooq, A.; Naeem, M.A.; Ahmad, A.; Liu, L. Structure and properties of high quality natural cellulose nano fibrils from a novel material Ficus natalensis barkcloth. J. Ind. Text. 2019, 1–17. [Google Scholar] [CrossRef]
- Mariano, M.; Cercená, R.; Soldi, V. Thermal characterization of cellulose nanocrystals isolated from sisal fibers using acid hydrolysis. Ind. Crop. Prod. 2016, 94, 454–462. [Google Scholar] [CrossRef]
- Zhang, N.; Tao, P.; Lu, Y.; Nie, S. Effect of lignin on the thermal stability of cellulose nanofibrils produced from bagasse pulp. Cellulose 2019, 26, 7823–7835. [Google Scholar] [CrossRef]
- Li, S.; Lee, P.S. Development and applications of transparent conductive nanocellulose paper. Sci. Technol. Adv. Mater. 2017, 18, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; He, M.; Zhou, Y.; Nie, S.; Wang, Y.; Hu, S.; Yang, H.; Li, H.; Tong, Y. Highly cuboid-shaped heterobimetallic metal-organic frameworks derived from porous Co/ZnO/C microrods with improved electromagnetic wave absorption capabilities. ACS Appl. Mater. Interfaces 2018, 10, 29136–29144. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Christov, N.; Cristobal, G.; Bourgaux, C.; Heux, L.; Boucenna, I.; Berret, J.F. Design of eco-friendly fabric softeners: Structure, rheology and interaction with cellulose nanocrystals. J. Colloid Interface Sci. 2018, 525, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Li, S.; Coumes, F.; Darcos, V.; Him, J.L.K.; Bron, P. Modeling and self-assembly behavior of PEG-PLA-PEG triblock copolymers in aqueous solution. Nanoscale 2013, 5, 9010–9017. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Wu, Z.; Xing, C.; Zhang, Q.; Wei, Z.; Nie, S. Effect of enzymatic treatment on the thermal stability of cellulose nanofibrils. Cellulose 2019, 26, 7717–7725. [Google Scholar] [CrossRef]
- Lin, X.; Wu, Z.; Zhang, C.; Liu, S.; Nie, S. Enzymatic pulping of lignocellulosic biomass. Ind. Crop. Prod. 2018, 120, 16–24. [Google Scholar] [CrossRef]
- Huang, C.; Dong, H.; Su, Y.; Wu, Y.; Narron, R.; Yong, Q. Synthesis of carbon quantum dot nanoparticles derived from byproducts in bio-refinery process for cell imaging and in vivo bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atifi, S.; Miao, C.; Hamad, W.Y. Surface modification of lignin for applications in polypropylene blends. J. Appl. Polym. Sci. 2017, 134, 45103–45112. [Google Scholar] [CrossRef]
- Cusola, O.; Rojas, O.J.; Roncero, M.B. Lignin particles for multifunctional membranes, antioxidative microfiltration, patterning, and 3D structuring. ACS Appl. Mater. Interfaces 2019, 11, 45226–45236. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Rajasekharan, S.K.; Lee, J. K-carrageenan/lignin composite films: Biofilm inhibition, antioxidant activity, cytocompatibility, UV and water barrier properties. Mater. Today Commun. 2020, 24, 101346–101355. [Google Scholar] [CrossRef]
- Nair, S.S.; Yan, N. Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 2015, 22, 3137–3150. [Google Scholar] [CrossRef]
- Vanholme, R.; Storme, V.; Vanholme, B. A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell 2012, 24, 3506–3529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeMartini, J.D.; Pattathil, S.; Miller, J.S. Investigating plant cell wall components that affect biomass recalcitrance in poplar and switchgrass. Energy Environ. Sci. 2013, 6, 898–909. [Google Scholar] [CrossRef]
- Isaac, A.; de Paula, J.; Viana, C.M. From nano- to micrometer scale: The role of microwave-assisted acid and alkali pretreatments in the sugarcane biomass structure. Biotechnol. Biofuels 2018, 11, 73. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Mathew, L.; Thomas, S. Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev. Adv. Mater. Sci. 2014, 37, 20–28. [Google Scholar]
- Qing, Y.; Yi, J.; Wu, Y.; Cai, Z.; Wu, Q. Research progress in cellulose nanofiber films. Trans. China Pulp. Pap. 2016, 31, 55–62. [Google Scholar]
- Lê, H.Q.; Dimic-Misic, K.; Johansson, L.; Maloney, T.; Sixta, H. Effect of lignin on the morphology and rheological properties of nanofibrillated cellulose produced from γ-valerolactone/water fractionation process. Cellulose 2018, 25, 179–194. [Google Scholar] [CrossRef]
- Herrera, M.; Thitiwutthisakul, K.; Yang, X.; Rujitanaroj, P.; Rojas, R.; Berglund, L. Preparation and evaluation of high-lignin content cellulose nanofibrils from eucalyptus pulp. Cellulose 2018, 25, 3121–3133. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Liu, X.; Yang, Q.; Song, X.; Qin, C.; Wang, S.; Li, K. Effects of residual lignin on composition, structure and properties of mechanically defibrillated cellulose fibrils and films. Cellulose 2019, 26, 1577–1593. [Google Scholar] [CrossRef]
- Wongjaiyen, T.; Brostow, W.; Chonkaew, W. Tensile properties and wear resistance of epoxy nanocomposites reinforced with cellulose nanofibers. Polym. Bull. 2017, 75, 2039–2051. [Google Scholar] [CrossRef]
- Zainuddin, S.Y.; Ahmad, I.; Kargarzadeh, H. Potential of using multiscale kenaf fibers as reinforcing filler in cassava starch-kenaf biocomposites. Carbohydr. Polym. 2013, 92, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, C.; Luo, B. Comparing impacts of physicochemical properties and hydrolytic inhibitors on enzymatic hydrolysis of sugarcane bagasse. Bioprocess Biosyst. Eng. 2020, 43, 111–122. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, W.; Gu, F.; Cao, T.; Jin, Y. Comparison of the substrate enzymatic digestibility and lignin structure of wheat straw stems and leaves pretreated by green liquor. Bioresour. Technol. 2016, 199, 181–187. [Google Scholar] [CrossRef] [PubMed]
- BH Sluiter, A.; Hames, B.; Ruiz-Peinado, R.; Scarlata, C.; Sluiter, W.J.; Templaton, D.; Crocker, A.; Sluiter, M.D. Determination of structural carbohydrates and lignin in biomass. Natl. Renew. Energy Lab. 2008, 510, 1–16. [Google Scholar]
- Herrera-Morales, J.; Turley, T.A.; Betancourt-Ponce, M.; Nicolau, E. Nanocellulose-block copolymer films for the removal of emerging organic contaminants from aqueous solutions. Materials 2019, 12, 230. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.W.; Hasanulbasori, M.A.; Chiat, P.F.; Lee, H.V. Pyrus pyrifolia fruit peel as sustainable source for spherical and porous network based nanocellulose synthesis via one-pot hydrolysis system. Int. J. Biol. Macromol. 2019, 123, 1305–1319. [Google Scholar] [CrossRef]
- Yue, Z.; Hou, Q.; Liu, W.; Yu, S. Autohydrolysis prior to poplar chemi-mechanical pulping: Impact of surface lignin on subsequent alkali impregnation. Bioresour. Technol. 2019, 282, 318–324. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Bian, H.; Gao, Y.; Wang, R.; Liu, Z.; Wu, W.; Dai, H. Contribution of lignin to the surface structure and physical performance of cellulose nanofibrils film. Cellulose 2018, 25, 1309–1318. [Google Scholar] [CrossRef]
- Errokh, A.; Magnin, A.; Putaux, J.L.; Boufi, S. Hybrid nanocellulose decorated with silver nanoparticles as reinforcing filler with antibacterial properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110044. [Google Scholar] [CrossRef]
- Hong, S.; Song, Y.; Yuan, Y. Production and characterization of lignin containing nanocellulose from luffa through an acidic deep eutectic solvent treatment and systematic fractionation. Ind. Crop. Prod. 2020, 143, 111913. [Google Scholar] [CrossRef]
- Sim, S.F.; Mohamed, M.; Lu, N.; Sarman, N.S.P.; Samsudin, S.N.S. Computer-assisted analysis of fourier transform infrared (ftir) spectra for characterization of various treated and untreated agriculture biomass. Bioresources 2012, 7, 5367–5380. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Zhang, Y.; Li, W.; Xu, X.; Zhang, H.; Zhao, Y.; Lin, J.; Sun, D. Facile synthesis and light-induced antibacterial activity of ketoprofen functionalized bacterial cellulose membranes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 231–238. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Tan, S.; Gao, J.; Fu, Y.; Liu, Z. Hydrothermal synthesis of Ag nanoparticles on the nanocellulose and their antibacterial study. Inorg. Chem. Commun. 2019, 100, 44–50. [Google Scholar] [CrossRef]
- Hsu, T.C.; Guo, G.L.; Chen, W.H.; Hwang, W.S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, F.; Jin, L.; Liu, B.; Mao, Y.; Liu, Y.; Huang, J. Preparation of spherical nanocellulose from waste paper by aqueous NaOH/thiourea. Cellulose 2019, 26, 5177–5185. [Google Scholar] [CrossRef]
- Hamed, S.A.A.K.M.; Hassan, M.L. A new mixture of hydroxypropyl cellulose and nanocellulose for wood consolidation. J. Cult. Herit. 2019, 35, 140–144. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Synthesis of phosphoric acid-treated sugarcane bagasse cellulose nanocrystal and its thermal properties enhancement for poly(lactic acid) nanocomposites. J. Thermoplast. Compos. Mater. 2018, 32, 619–634. [Google Scholar] [CrossRef]
- Li, M.; Yin, J.; Hu, L.; Chen, S.; Min, D.; Wang, S.; Luo, L. Effect of hydrogen peroxide bleaching on anionic groups and structures of sulfonated chemo-mechanical pulp fibers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124060–124068. [Google Scholar] [CrossRef]
- Koljonen, K.; Österberg, M.; Johansson, L.S.; Stenius, P. Surface chemistry and morphology of different mechanical pulps determined by ESCA and AFM. Colloids Surf. A Physicochem. Eng. Asp. 2003, 228, 143–158. [Google Scholar] [CrossRef]
- Hon, D.N.-S. ESCA study of oxidized wood surfaces. J. Appl. Polym. Sci. 1984, 2777–2784. [Google Scholar] [CrossRef]
- Supian, M.A.F.; Amin, K.N.M.; Jamari, S.S.; Mohamad, S. Production of cellulose nanofiber (CNF) from empty fruit bunch (EFB) via mechanical method. J. Environ. Chem. Eng. 2020, 8, 103020–103024. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Gwon, J.G.; Lee, S.Y.; Doh, G.H.; Kim, J.H. Characterization of chemically modified wood fibers using FTIR spectroscopy for biocomposites. J. Appl. Polym. Sci. 2010, 116, 3212–3219. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16702–16709. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Nair, S.S.; Chen, H.; Yan, N.; Cao, J. Effects of lignin content on mechanical and thermal properties of polypropylene composites reinforced with micro particles of spray dried cellulose nanofibrils. ACS Sustain. Chem. Eng. 2018, 6, 11078–11086. [Google Scholar] [CrossRef]
- Hambardzumyan, A.; Foulon, L.; Chabbert, B.; Aguie-Beghin, V. Natural organic UV-absorbent coatings based on cellulose and lignin: Designed effects on spectroscopic properties. Biomacromolecules 2012, 13, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]








| Samples | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| SCB | 41.7 ± 0.6 | 26.6 ± 0.4 | 24.4 ± 0.7 |
| SCB-HW | 45.4 ± 0.6 | 5.5 ± 0.6 | 22.5 ± 0.6 |
| SCB-GL | 46.9 ± 0.5 | 7.6 ± 0.4 | 8.4 ± 0.3 |
| SCB-SC | 62.6 ± 0.3 | 3.5 ± 0.6 | 1.8 ± 0.3 |
| SP | 43.6 ± 0.2 | 23.9 ± 0.6 | 28.6 ± 0.7 |
| SP-HW | 47.4 ± 0.2 | 7.5 ± 0.6 | 26.0 ± 0.4 |
| SP-GL | 49.2 ± 0.2 | 9.1 ± 0.5 | 15.1 ± 0.7 |
| SP-SC | 55.6 ± 0.4 | 4.0 ± 0.5 | 2.8 ± 0.6 |
| Samples | C (%) | O (%) | S (%) | O/C | SLC (%) |
|---|---|---|---|---|---|
| SCB-HW | 69.4 | 30.4 | 0.17 | 0.44 | 78 |
| SCB-GL | 66.5 | 33.2 | 0.23 | 0.50 | 66 |
| SCB-SC | 64.8 | 35.0 | 0.19 | 0.54 | 58 |
| SP-HW | 71.6 | 28.1 | 0.15 | 0.39 | 87 |
| SP-GL | 68.2 | 31.6 | 0.26 | 0.46 | 74 |
| SP-SC | 59.1 | 40.6 | 0.20 | 0.69 | 28 |
| Samples | C1 (%) | C2 (%) | C3 (%) |
|---|---|---|---|
| SCB-HW | 48.5 | 37.5 | 13.9 |
| SCB-GL | 39.5 | 42.4 | 18.2 |
| SCB-SC | 33.7 | 46.1 | 20.2 |
| SP-HW | 54.4 | 34.1 | 11.5 |
| SP-GL | 42.5 | 40.3 | 17.2 |
| SP-SC | 21.9 | 54.7 | 23.4 |
| Samples | CrI (%) |
|---|---|
| SCB-HW | 54.7 ± 0.5 |
| SCB-GL | 61.4 ± 0.7 |
| SCB-SC | 67.1 ± 0.4 |
| SP-HW | 51.2 ± 0.8 |
| SP-GL | 63.1 ± 0.4 |
| SP-SC | 65.5 ± 0.6 |
| Sample | Region Ⅰ | Region Ⅱ | Region Ⅲ | Tonset (°C) | Td (°C) | ||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Residual Moisture (wt.%) | Temperature (°C) | Mass Residue (wt.%) | Mass Residue (wt.%) | |||
| SCB-HW | 251 | 5.11 | 387 | 38.11 | 10.63 | 297 | 352 |
| SCB-GL | 243 | 5.78 | 382 | 37.06 | 11.59 | 291 | 346 |
| SCB-SC | 255 | 6.51 | 369 | 37.23 | 14.11 | 284 | 323 |
| SP-HW | 252 | 5.50 | 392 | 32.86 | 10.20 | 321 | 364 |
| SP-GL | 240 | 5.45 | 385 | 33.97 | 9.13 | 320 | 360 |
| SP-SC | 239 | 5.49 | 372 | 35.68 | 10.87 | 301 | 333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Wang, X.; Zhang, S.; Yuan, X.; Li, M.; Wang, S. Contribution of Different Pretreatments to the Thermal Stability and UV Resistance Performance of Cellulose Nanofiber Films. Coatings 2021, 11, 247. https://doi.org/10.3390/coatings11020247
Luo L, Wang X, Zhang S, Yuan X, Li M, Wang S. Contribution of Different Pretreatments to the Thermal Stability and UV Resistance Performance of Cellulose Nanofiber Films. Coatings. 2021; 11(2):247. https://doi.org/10.3390/coatings11020247
Chicago/Turabian StyleLuo, Lianxin, Xuchong Wang, Sheng Zhang, Xiaojun Yuan, Mingfu Li, and Shuangfei Wang. 2021. "Contribution of Different Pretreatments to the Thermal Stability and UV Resistance Performance of Cellulose Nanofiber Films" Coatings 11, no. 2: 247. https://doi.org/10.3390/coatings11020247