A Novel Decarburizing-Nitriding Treatment of Carburized/through-Hardened Bearing Steel towards Enhanced Nitriding Kinetics and Microstructure Refinement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermochemical Surface Treatment
- (1)
- Gas carburizing and decarburizing for M50NiLFor M50NiL steel, a “boost-diffusion” gas carburizing and a short decarburizing step were performed at continuous gas carburizing furnace (FAW Group) in Changchun, China, with the process schematically shown in Figure 1a. For comparison, a group of as-carburized samples (denoted as ‘C’) were kept at the same temperature and time with decarburizing while the atmosphere maintained with the carbon potential of Cp = 0.85%.
- (2)
- Decarburizing and tempering for M50To decarburize M50, specimens were solution treated in a box furnace in air at 1100 °C for 20 min. Then the specimens were encapsulated in a vacuum quartz tube and tempered at 520 °C for 4 h. The duplex process is schematically shown in Figure 1b. For comparison, a “Blank” sample was solution treated in vacuum, followed by tempering at the same condition as the decarburized sample. It should be noted that the oxidized layers of air-decarburized samples were grinded off prior to plasma nitriding.
- (3)
- Plasma nitridingPlasma nitriding was performed in a 30-kW pulse plasma multi-element furnace (LDMC-30AFZ, 30 kW). The atmospheric pressure was 200 Pa, and the voltage was kept at 650 V. The heating rate was 4 °C/min. M50NiL steel samples were nitrided at 520 °C, 540 °C and 580 °C for 4, 8 and 16 h in a gas mixture of N2 and H2 with a flow ratio 1:1. M50 steel samples were nitrided at 500 °C, 520 °C and 540 °C for 4, 8 and 12 h in a gas mixture of N2 and H2 with a flow ratio 1:3. After nitriding, the specimens were furnace cooled to room temperature in N2 flow with a cooling rate of ~2 °C/min.
2.3. Microstructure Characterizations
3. Results and Discussion
3.1. Microstructures of Modified Surface Layers of M50NiL Steel
3.2. Microstructures of Modified Surface Layers of M50 Steel
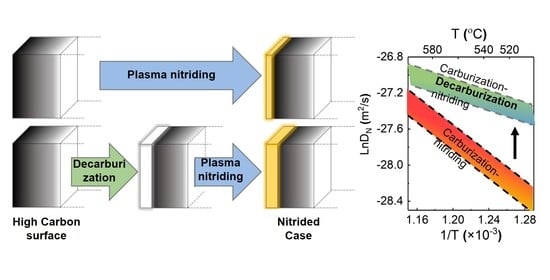
3.3. The Kinetics of Nitrogen Diffusion during Plasma Nitriding
4. Conclusions
- (1)
- Compared with the conventional “duplex surface engine” method, the duplex process with decarburizing step performed in this work can significantly increase the nitrided layer thickness even by more than ~100% and the surface hardness reach ~1200 HV0.1.
- (2)
- The analysis of nitriding kinetics shows that the pre-existing carbon atoms solutioned in Fe lattice can hinder the growth of nitride layers. Decarburization performed in air can produce a decarburized case with less carbon and residual compressive stresses, which can significantly increase the thickness of the subsequent nitride layer.
- (3)
- Low-nitrogen compound FeN0.076 with high hardness was produced on the modified surface of the decarburizing sample, which indicates that decarburizing can promote the occurrence of surface microstructure refinement via spinodal decomposition during plasma nitriding. Under the same nitriding conditions, the nanostructure can enhance nitrogen diffusion into the matrix along grain boundaries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhadeshia, H. Steels for bearings. Prog. Mater. Sci. 2012, 57, 268–435. [Google Scholar] [CrossRef]
- Flodström, I. Nitrocarburizing and High Temperature Nitriding of Steels in Bearing Applications; Chalmers University of Technology: Göteborg, Sweden, 2012; pp. 34–36. [Google Scholar]
- Ooi, S.; Bhadeshia, H.K.D.H. Duplex Hardening of Steels for Aeroengine Bearings. ISIJ Int. 2012, 52, 1927–1934. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, M.; Johnson, M.; Miedema, K.; Scheetz, J.; Williams, J. Advances in Steel Technologies for Rolling Bearings. Introduction of Nitrided M50 and M50NiL Bearings into Jet Engine Mainshaft Applications. In Bearing Steel Technologies; ASTM International: Washington, DC, USA, 2015; Volume 10. [Google Scholar]
- Streit, E.; Brock, J.; Poulin, P. Performance Evaluation of “Duplex Hardened” Bearings for Advanced Turbine Engine Applications. J. ASTM Int. 2006, 3, 1–9. [Google Scholar] [CrossRef]
- Bell, T.; Dong, H.; Sun, Y. Realising the potential of duplex surface engineering. Tribol. Int. 1998, 31, 127–137. [Google Scholar] [CrossRef]
- Davies, D. Duplex Hardening: An Advanced Surface Engineering Technique for Helicopter Transmissions. In Surface Engineering; Springer: Berlin/Heidelberg, Germany, 1990; pp. 228–237. [Google Scholar]
- Tsujikawa, M.; Yamauchi, N.; Ueda, N.; Sone, T.; Hirose, Y. Behavior of carbon in low temperature plasma nitriding layer of austenitic stainless steel. Surf. Coat. Technol. 2005, 193, 309–313. [Google Scholar] [CrossRef]
- Duarte, M.C.; Godoy, C.; Wilson, J.A.-B.; Godoy, G. Analysis of sliding wear tests of plasma processed AISI 316L steel. Surf. Coat. Technol. 2014, 260, 316–325. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Yoshida, D.; Yamauchi, N.; Ueda, N.; Sone, T. Surface Modification of SUS304 Stainless Steel Using Carbon Push-Ahead Effect by Low Temperature Plasma Nitriding. Mater. Trans. 2005, 46, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Tsujikawa, M.; Yoshida, D.; Yamauchi, N.; Ueda, N.; Sone, T.; Tanaka, S. Surface material design of 316 stainless steel by combination of low temperature carburizing and nitriding. Surf. Coat. Technol. 2005, 200, 507–511. [Google Scholar] [CrossRef]
- Bloyce, A.; Sun, Y.; Li, X. Duplex thermochemical processing of M 50 NiL for gear applications. Heat Treat. Met. Birm. 1999, 26, 37–40. [Google Scholar]
- Egert, P.; Maliska, A.; Silva, H.; Speller, C. Decarburization during plasma nitriding. Surf. Coat. Technol. 1999, 122, 33–38. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, J.; Wang, H.; Wen, Z.; Liu, Q.; Zhao, G.; Wang, W. Effects of decarburization on the wear resistance and damage mechanisms of rail steels subject to contact fatigue. Wear 2016, 364, 130–143. [Google Scholar] [CrossRef]
- Hussain, K.; Tauqir, A.; Haq, A.U.; Khan, A.Q. Effect of retained austenite on gas nitriding of high strength steel. Mater. Sci. Technol. 1998, 14, 1218–1220. [Google Scholar] [CrossRef]
- Akbari, A.; Mohammadzadeh, R.; Templier, C.; Riviere, J. Effect of the initial microstructure on the plasma nitriding behavior of AISI M2 high speed steel. Surf. Coat. Technol. 2010, 204, 4114–4120. [Google Scholar] [CrossRef]
- Calliari, I.; Dabalà, M.; Ramous, E.; Zanesco, M.; Gianotti, E. Microstructure of a Nitrided Steel Previously Decarburized. J. Mater. Eng. Perform. 2006, 15, 693–698. [Google Scholar] [CrossRef]
- Yan, M.; Chen, B.; Li, B. Microstructure and mechanical properties from an attractive combination of plasma nitriding and secondary hardening of M50 steel. Appl. Surf. Sci. 2018, 455, 1–7. [Google Scholar] [CrossRef]
- Wang, J.; Zou, H.; Li, C.; Qiu, S.; Shen, B. The spinodal decomposition in 17-4PH stainless steel subjected to long-term aging at 350 °C. Mater. Charact. 2008, 59, 587–591. [Google Scholar] [CrossRef]
- Yao, J.; Yan, F.; Yan, M.; Zhang, Y.; Huang, D.; Xu, Y. The mechanism of surface nanocrystallization during plasma nitriding. Appl. Surf. Sci. 2019, 488, 462–467. [Google Scholar] [CrossRef]
- Sasidhar, K.N.; Meka, S.R. Thermodynamic reasoning for colossal N supersaturation in austenitic and ferritic stainless steels during low-temperature nitridation. Sci. Rep. 2019, 9, 7996. [Google Scholar] [CrossRef]
- Sun, Y.; Bell, T. A numerical model of plasma nitriding of low alloy steels. Mater. Sci. Eng. A 1997, 224, 33–47. [Google Scholar] [CrossRef]
- Zagonel, L.F.; Figueroa, C.A.; Droppa, R.; Alvarez, F. Influence of the process temperature on the steel microstructure and hardening in pulsed plasma nitriding. Surf. Coat. Technol. 2006, 201, 452–457. [Google Scholar] [CrossRef]
- Scheuer, C.; Cardoso, R.; Mafra, M.; Brunatto, S. AISI 420 martensitic stainless steel low-temperature plasma assisted carburizing kinetics. Surf. Coat. Technol. 2013, 214, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yan, M.; Zhang, S.; Guo, J.; Jiang, S.; Li, D. Diffusion behavior of carbon and its hardening effect on plasma carburized M50NiL steel: Influences of treatment temperature and duration. Surf. Coat. Technol. 2018, 333, 96–103. [Google Scholar] [CrossRef]
- Pinedo, C.E.; Monteiro, W.A. On the kinetics of plasma nitriding a martensitic stainless steel type AISI 420. Surf. Coat. Technol. 2004, 179, 119–123. [Google Scholar] [CrossRef]






| Sample Denotation | Carburizing | Decarburizing | Plasma Nitriding | |
|---|---|---|---|---|
| Boost | Diffusion | |||
| C | 930 °C, 430 min, Cp = 1.15% | 910 °C, 280 min, Cp = 0.85% | 850 °C, 30 min, Cp = 0.85% | - |
| CD | 850 °C, 30 min, air | |||
| C-N | 850 °C, 30 min, Cp = 0.85% | 520 °C, 540 °C, 580 °C 4 h, 8 h, 12 h N2:H2 = 1:1 | ||
| CD-N | 850 °C, 30 min, air | |||
| Sample Denotation | Solutioning/Decarburizing | Tempering | Plasma Nitriding |
|---|---|---|---|
| Blank | 1100 °C, 20 min, vacuum | 520 °C, 4 h, vacuum | - |
| D | 1100 °C, 20 min, air | ||
| N | 1100 °C, 20 min, vacuum | 500 °C, 520 °C, 540 °C 4 h, 8 h, 12 h N2:H2 = 1:3 | |
| D-N | 1100 °C, 20 min, air |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, F.; Yao, J.; Chen, B.; Yang, Y.; Xu, Y.; Yan, M.; Zhang, Y. A Novel Decarburizing-Nitriding Treatment of Carburized/through-Hardened Bearing Steel towards Enhanced Nitriding Kinetics and Microstructure Refinement. Coatings 2021, 11, 112. https://doi.org/10.3390/coatings11020112
Yan F, Yao J, Chen B, Yang Y, Xu Y, Yan M, Zhang Y. A Novel Decarburizing-Nitriding Treatment of Carburized/through-Hardened Bearing Steel towards Enhanced Nitriding Kinetics and Microstructure Refinement. Coatings. 2021; 11(2):112. https://doi.org/10.3390/coatings11020112
Chicago/Turabian StyleYan, Fuyao, Jiawei Yao, Baofeng Chen, Ying Yang, Yueming Xu, Mufu Yan, and Yanxiang Zhang. 2021. "A Novel Decarburizing-Nitriding Treatment of Carburized/through-Hardened Bearing Steel towards Enhanced Nitriding Kinetics and Microstructure Refinement" Coatings 11, no. 2: 112. https://doi.org/10.3390/coatings11020112