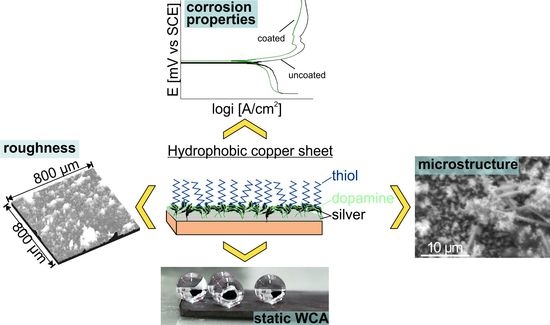
Deposition of Super-Hydrophobic Silver Film on Copper Substrate and Evaluation of Its Corrosion Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Hydrophobic Coating
2.2. Characterization of Modified Copper Surfaces
2.3. Corrosion Behavior of Silver-Coated Copper Surfaces
3. Results and Discussion
3.1. Influence of Various Parameters in the Synthesis Process of the Hydrophobic Coating
3.2. Surface Morphology and Wettability of the Prepared Hydrophobic Coatings
3.3. Electrochemical Corrosion Results
3.3.1. Polarization Measurements
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3.3. Proposed Equivalent Electrical Circuit Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable Superhydrophobic and superamphiphobic polymeric surfaces and their applications: A review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and engineering of superhydrophobic surfaces: Review of corrosion resistance, chemical and mechanical stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired surfaces with superwettability: New Insight on theory, design, and applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, L. Metallic Surfaces with Special Wettability. Nanoscale 2011, 3, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Vanithakumari, S.C.; Choubey, A.K.; Thinaharan, C.; Gupta, R.K.; George, R.P.; Kaul, R.; Bindra, K.S.; Philip, J. Laser patterned titanium surfaces with superior antibiofouling, superhydrophobicity, self-cleaning and durability: Role of line spacing. Surf. Coat. Technol. 2021, 418, 127257. [Google Scholar] [CrossRef]
- Wu, S.-T.; Huang, C.-Y.; Weng, C.-C.; Chang, C.-C.; Li, B.-R.; Hsu, C.-S. Rapid prototyping of an open-surface microfluidic platform using wettability-patterned surfaces prepared by an atmospheric-pressure plasma jet. ACS Omega 2019, 4, 16292–16299. [Google Scholar] [CrossRef] [Green Version]
- Bi, P.; Li, H.; Zhao, G.; Ran, M.; Cao, L.; Guo, H.; Xue, Y. Robust super-hydrophobic coating prepared by electrochemical surface engineering for corrosion protection. Coatings 2019, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Dimitrakellis, P.; Gogolides, E. Hydrophobic and superhydrophobic surfaces fabricated using atmospheric pressure cold plasma technology: A review. Adv. Colloid Interface Sci. 2018, 254, 1–21. [Google Scholar] [CrossRef]
- Hafiz, M.A.; Muhammad, A.Q.; Sullahuddin, M.; Ghulam, M. Techniques for the Fabrication of Super-Hydrophobic Surfaces and Their Heat Transfer Applications. In Heat Transfer—Models, Methods and Applications; Konstantin, V., Ed.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/58728 (accessed on 12 May 2021).
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Liu, L.; Chen, R.; Liu, W.; Zhang, Y.; Shi, X.; Pan, Q. Fabrication of superhydrophobic copper sulfide film for corrosion protection of copper. Surf. Coat. Technol. 2015, 272, 221–228. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, L.; Qiang, X.; Liu, Y.; Sun, Z.; Wang, B. Fabrication of superhydrophobic copper surface with excellent corrosion resistance. Appl. Phys. A Mater. Sci. Process. 2015, 119, 75–83. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, H.; Lin, F.; Wang, Y.; Wang, L.; Dong, Y.; Li, W. Superhydrophobic surface fabricated on iron substrate by black chromium electrodeposition and its corrosion resistance property. Appl. Surf. Sci. 2016, 378, 388–396. [Google Scholar] [CrossRef]
- Chun, D.M.; Ngo, C.V.; Lee, K.M. Fast Fabrication of superhydrophobic metallic surface using nanosecond laser texturing and low-temperature annealing. CIRP Ann.-Manuf. Technol. 2016, 65, 519–522. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Z.; Liang, J.; Wang, Y.; Chen, H. Preparation of superhydrophobic films on copper substrate for corrosion protection. Surf. Coat. Technol. 2014, 244, 1–8. [Google Scholar] [CrossRef]
- Wang, P.; Qiu, R.; Zhang, D.; Lin, Z.; Hou, B. Fabricated Super-Hydrophobic Film with Potentiostatic Electrolysis Method on Copper for Corrosion Protection. Electrochim. Acta 2010, 56, 517–522. [Google Scholar] [CrossRef]
- Talesh Bahrami, H.R.; Ahmadi, B.; Saffari, H. Optimal Condition for Fabricating Superhydrophobic Copper Surfaces with Controlled Oxidation and Modification Processes. Mater. Lett. 2017, 189, 62–65. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, M.; Liu, W.; Shen, X.X.; Min, Y.; Xu, Q. The research on preparation of superhydrophobic surfaces of pure copper by hydrothermal method and its corrosion resistance. Electrochim. Acta 2018, 270, 310–318. [Google Scholar] [CrossRef]
- Feng, L.; Wang, J.; Shi, X.; Chai, C. Superhydrophobic copper surface with mechanical, chemical, and UV durability along with corrosion resistance and self-cleaning effect. Appl. Phys. A Mater. Sci. Process. 2019, 125, 261. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Wan, Y.; Wu, J. Green approach to fabrication of a super-hydrophobic film on copper and the consequent corrosion resistance. Corros. Sci. 2014, 80, 366–373. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, J.; Liu, J.; Han, Z.; Ren, L. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coatings on copper substrate. Corros. Sci. 2015, 94, 190–196. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Q.; Han, J.; Chen, X.; Min, Y. A novel combination approach for the preparation of superhydrophobic surface on copper and the consequent corrosion resistance. Corros. Sci. 2016, 110, 105–113. [Google Scholar] [CrossRef]
- Mousavi, S.M.A.; Pitchumani, R. A Study of corrosion on electrodeposited superhydrophobic copper surfaces. Corros. Sci. 2021, 186, 109420. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Gallant, D.; Chen, X.G. Corrosion resistance properties of superhydrophobic copper surfaces fabricated by one-step electrochemical modification process. Appl. Surf. Sci. 2013, 282, 689–694. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Hu, Z.; Zhang, X.; Wu, S.; Wang, R.; Zhu, Y. A Novel Electrodeposition route for fabrication of the superhydrophobic surface with unique self-cleaning, mechanical abrasion and corrosion resistance properties. Chem. Eng. J. 2016, 303, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurface Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J. Chemistry of polydopamine—scope, variation, and limitation. Eur J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- ASTM G69-20, Standard Test Method for Measurement of Corrosion Potentials of Aluminum Alloys; ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM G71-81, Standard Guide for Conducting and Evaluating Galvanic Corrosion Tests in Electrolytes; ASTM International: West Conshohocken, PA, USA, 2019.
- Lakshminarayanan, R.; Madhavi, S.; Poh Choo Sim, C. Oxidative polymerization of dopamine: A High-Definition Multifunctional Coatings for Electrospun Nanofibers In Dopamine—Health and Disease; Yenisetti, S.C., Ed.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/63723 (accessed on 12 May 2021). [CrossRef] [Green Version]
- Qiu, W.Z.; Yang, H.C.; Xu, Z.K. Dopamine-Assisted Co-Deposition: An emerging and promising strategy for surface modification. Adv. Colloid Interface Sci. 2018, 256, 111–125. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Shang, B.; Wen, P.; Peng, B.; Deng, Z. Bioinspired one-step construction of hierarchical superhydrophobic surfaces for oil/water separation. J. Colloid Interface Sci. 2018, 531, 300–310. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Luchini, A.; Napolitano, A.; Derrico, G.; Vitiello, G.; Szekely, N.; Dischia, M.; Paduano, L. Tris buffer modulates polydopamine growth, aggregation, and paramagnetic properties. Langmuir 2014, 30, 9811–9818. [Google Scholar] [CrossRef]
- Feng, L.; Yang, M.; Shi, X.; Liu, Y.; Wang, Y.; Qiang, X. Colloids and surfaces A: Physicochemical and engineering aspects copper-based superhydrophobic materials with long-term durability, stability, regenerability, and self-cleaning property. Physicochem. Eng. Asp. 2016, 508, 39–47. [Google Scholar] [CrossRef]
- Boscher, N.D.; Vaché, V.; Carminati, P.; Grysan, P.; Choquet, P. A Simple and scalable approach towards the preparation of superhydrophobic surfaces-importance of the surface roughness skewness. J. Mater. Chem. A 2014, 2, 5744–5750. [Google Scholar] [CrossRef]
- Che, P.; Liu, W.; Chang, X.; Wang, A.; Han, Y. Multifunctional silver film with superhydrophobic and antibacterial properties. Nano Res. 2016, 9, 442–450. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Son, Y.; Zhang, W.; Spindler, L.M.; Han, Z.; Ren, L. A smart switchable bioinspired copper foam responding to different ph droplets for reversible oil-water separation. J. Mater. Chem. A 2017, 5, 2603–2612. [Google Scholar] [CrossRef]
- Zhou, W.; Li, G.; Wang, L.; Chen, Z.; Lin, Y. A Facile Method for the Fabrication of a superhydrophobic polydopamine-coated copper foam for oil/water separation. Appl. Surf. Sci. 2017, 413, 140–148. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Yang, G.; Yu, L.; Zhang, P. Preparation of silver-cuprous oxide/stearic acid composite coating with superhydrophobicity on copper substrate and evaluation of its friction-reducing and anticorrosion abilities. Appl. Surf. Sci. 2014, 289, 21–26. [Google Scholar] [CrossRef]
- Safaee, A.; Sarkar, D.K.; Farzaneh, M. Superhydrophobic properties of silver-coated films on copper surface by galvanic exchange reaction. Appl. Surf. Sci. 2008, 254, 2493–2498. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Dai, L. Novel silver nanostructures from silver mirror reaction on reactive substrates. J. Phys. Chem. B 2005, 109, 13985–13990. [Google Scholar] [CrossRef]
- Fang, J.; You, H.; Kong, P.; Yi, Y.; Song, X.; Ding, B. Dendritic silver nanostructure growth and evolution in replacement reaction. Cryst. Growth Des. 2007, 7, 864–867. [Google Scholar] [CrossRef]
- Tu, S.H.; Wu, H.C.; Wu, C.J.; Cheng, S.L.; Sheng, Y.J.; Tsao, H.K. Growing hydrophobicity on a smooth copper oxide thin film at room temperature and reversible wettability transition. Appl. Surf. Sci. 2014, 316, 88–92. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, K.S.; Pippel, E.; Kim, S.; Kim, J.H.; Lee, H.J. Facile route toward mechanically stable superhydrophobic copper using oxidation-reduction induced morphology changes. J. Phys. Chem. C 2012, 116, 2781–2790. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Stergioudi, F.; Vogiatzis, C.A.; Pavlidou, E.; Skolianos, S.; Michailidis, N. Corrosion resistance of porous NiTi biomedical alloy in simulated body fluids. Smart Mater. Struct. 2016, 25, 095024. [Google Scholar] [CrossRef]
- Stergioudi, F.; Vogiatzis, C.A.; Gkrekos, K.; Michailidis, N.; Skolianos, S.M. Electrochemical corrosion evaluation of pure, carbon-coated and anodized Al foams. Corros. Sci. 2015, 91, 151–159. [Google Scholar] [CrossRef]









| Specimen | Ecorr [mV] | icorr [μA/cm2] | βc [mV] | βa [mV] | Rp [kohm/cm2] | Corrosion Rate μm/year |
|---|---|---|---|---|---|---|
| Copper substrate | −214 | 2 | 36.8 | −53.7 | 0.82 | 24.06 |
| Silver-coated copper | −170 | 0.5 | 38.3 | −49.8 | 10.41 | 15.961 |
| Material | Rs | Rpor | CPE1 | Rct | CPE2 | Zw | Ro | CPE3 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ohm cm2 | Ohm cm2 | F × sα−1 | α1 | Ohm cm2 | F × sα−1 | α2 | Ohm s−0.5 | Ohm cm2 | F sα−1 | α3 | |
| Copper substrate | 6.57 | 721 | 67 × 10−6 | 0.82 | 717 | 228 × 10−6 | 0.99 | 148 | - | - | - |
| Coated copper | 22.31 | 5446 | 110 × 10−6 | 0.89 | 2229 | 1.96 × 10−6 | 0.53 | - | 18.76 | 155 × 10−6 | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stergioudi, F.; Baxevani, A.; Mavropoulos, A.; Skordaris, G. Deposition of Super-Hydrophobic Silver Film on Copper Substrate and Evaluation of Its Corrosion Properties. Coatings 2021, 11, 1299. https://doi.org/10.3390/coatings11111299
Stergioudi F, Baxevani A, Mavropoulos A, Skordaris G. Deposition of Super-Hydrophobic Silver Film on Copper Substrate and Evaluation of Its Corrosion Properties. Coatings. 2021; 11(11):1299. https://doi.org/10.3390/coatings11111299
Chicago/Turabian StyleStergioudi, Fani, Aikaterini Baxevani, Azarias Mavropoulos, and Georgios Skordaris. 2021. "Deposition of Super-Hydrophobic Silver Film on Copper Substrate and Evaluation of Its Corrosion Properties" Coatings 11, no. 11: 1299. https://doi.org/10.3390/coatings11111299