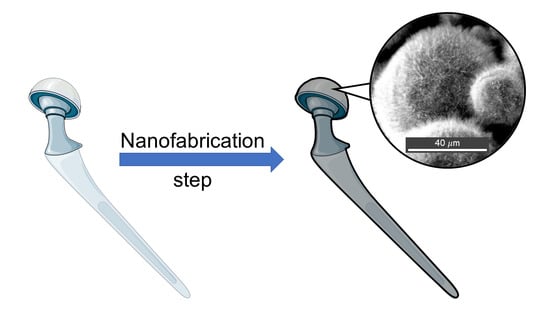
Protruding Nanostructured Surfaces for Antimicrobial and Osteogenic Titanium Implants
Abstract
:1. Introduction
Biofilm Formation on Medical Implants
| Medical Implant Type | Microbes Found | References |
|---|---|---|
| Orthopaedic Implants | Coagulase-negative staphylococci, haemolytic streptococci, enterococci, Proteus mirabilis, Bacteroides species, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa | [24,25] |
| Replacement Joints | S. aureus, S. epidermidis | [26] |
| Cardiac Pacemakers | S. aureus | [24] |
| Dental Implants | Gram-positive cocci (e.g., Streptococcus), Gram-negative anaerobic oral bacteria | [24,26] |
2. Prevention of Biofilm Formation Using Protruding Nanostructured Surfaces
3. Understanding Bacteria–Nanostructure Interactions
3.1. Stretching and Mechano-Inducing Models

3.2. Other Possible Bactericidal Mechanisms
4. Osteogenic Nanostructured Surfaces
| Influenced Process | Nanostructure | Related Genes/Pathways | References |
|---|---|---|---|
| Inflammation | Semispherical Protrusion | MCP-1 TNF-α | [67,68] |
| Cell attachment | Nanopillars Nanoislands | – | [69,70,71] |
| Osteogenesis | Nanoislands | Osteocalcin (OC), Osteopontin (OPN) | [69,72,73,75] |
| Nanopillars | Actin, Integrin, p38 MAPK and ERK Pathways | ||
| Osteoclastogenesis | Nanopillars | Osteoprotegerin (OPG), RANKL | [76] |
5. Fabricating Bactericidal and Osteogenic Nanostructures on Titanium Surfaces
5.1. Hydrothermal Process
5.2. Thermal Oxidation
5.3. Glancing Angle Deposition
5.4. Reactive Ion Etching
6. Discussion
6.1. Scalability and Applicability of Nanofabrication Techniques for Medical Implants
6.2. Eukaryotic Versus Prokaryotic Race for the Surface
6.3. Advantages and Disadvantages of Mechano-Biocidal Surfaces
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Webster, T.J. Nanotechnology for reducing orthopedic implant infections: Synthesis, characterization, and properties. In Orthopedic Biomaterials; Li, B., Webster, T., Eds.; Springer: Berlin, Germany, 2017; pp. 31–62. [Google Scholar]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and osseointegration properties of nanostructured titanium orthopaedic implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloys Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [Green Version]
- National Joint Registry for England, Wales, Northern Ireland and Isle of Man, 6th Annual Report 2019; National Joint Registry: London, UK, January 2019.
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional coatings and nanotopographies: Toward cell instructive and antibacterial implants. Adv. Healthc. Mater. 2019, 8, 1801103. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef]
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A., Jr. Biomaterial properties of titanium in dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Sargolzaie, N.; Moeintaghavi, A.; Shojaie, H. Comparing the quality of life of patients requesting dental implants before and after implant. Open Dent. J. 2017, 11, 485. [Google Scholar] [CrossRef] [Green Version]
- Catherine, D.; Koray, F.; Philip, F.; Stephen, H.; Craig, P.; David, S. A Dentist’s Guide To Implantology; The Association of Dental Implantology: Richmond, VA, USA, 2012. [Google Scholar]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors affecting the survival rate of dental implants: A retrospective study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351. [Google Scholar] [CrossRef]
- Passarelli, P.C.; De Leonardis, M.; Piccirillo, G.B.; Desantis, V.; Papa, R.; Rella, E.; Bonaviri, G.N.M.; Papi, P.; Pompa, G.; Pasquantonio, G.; et al. The effectiveness of chlorhexidine and air polishing system in the treatment of candida albicans infected dental implants: An experimental in vitro study. Antibiotics 2020, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Di Murro, B.; Papi, P.; Passarelli, P.C.; D’Addona, A.; Pompa, G. Attitude in radiographic post-operative assessment of dental implants among italian dentists: A cross-sectional survey. Antibiotics 2020, 9, 234. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, J.; Kolar, M.; Novotny, R.; Rihakova, P.; Tichá, V. Pathogenesis of prosthesis-related infection. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2003, 147, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burch, M.A.; Moriarty, T.F.; Kuehl, R.; Foster, A.; Morgenstern, M. Complications in orthopedic trauma surgery: Fracture-related infection. In Racing for the Surface; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer: Berlin, Germany, 2020; pp. 33–56. [Google Scholar]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.; Byren, I.; Atkins, B.L. The diagnosis and management of prosthetic joint infections. J. Antimicrob. Chemother. 2010, 65, iii45–iii54. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef]
- Joost, U.; Juganson, K.; Visnapuu, M.; Mortimer, M.; Kahru, A.; Nõmmiste, E.; Joost, U.; Kisand, V.; Ivask, A. Photocatalytic antibacterial activity of nano-TiO2 (anatase)-based thin films: Effects on Escherichia coli cells and fatty acids. J. Photochem. Photobiol. B Biol. 2015, 142, 178–185. [Google Scholar] [CrossRef]
- Stewart, P.S. Antimicrobial tolerance in biofilms. Microb. Biofilms 2015, 269–285. [Google Scholar] [CrossRef] [Green Version]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Darouiche, R.O.O.; Weinstein, R.A. Device-associated infections: A macroproblem that starts with microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K.D.V. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhushan, B.; Jung, Y.C.; Niemietz, A.; Koch, K. Lotus-like biomimetic hierarchical structures developed by the self-assembly of tubular plant waxes. Langmuir 2009, 25, 1659–1666. [Google Scholar] [CrossRef]
- Mann, E.E.; Manna, D.; Mettetal, M.R.; May, R.M.; Dannemiller, E.M.; Chung, K.K.; Brennan, A.B.; Reddy, S.T. Surface micropattern limits bacterial contamination. Antimicrob. Resist. Infect. Control 2014, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Sharklet. Sharklet—Products 2020. Available online: http://www.sharklet.com/our-products (accessed on 10 June 2020).
- Fernández, I.C.S.; Van der Mei, H.C.; Metzger, S.; Grainger, D.W.; Engelsman, A.F.; Nejadnik, M.R.; Busschera, H.J. In vitro and in vivo comparisons of staphylococcal biofilm formation on a cross-linked poly (ethylene glycol)-based polymer coating. Acta Biomater. 2010, 6, 1119–1124. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.T.; Kim, S.-K.; Lee, J.-H.; Choi, H.S.; Kim, C.-S.; Jeong, M.Y. Nanostructured multifunctional surface with antireflective and antimicrobial characteristics. ACS Appl. Mater. Interfaces 2015, 7, 326–331. [Google Scholar] [CrossRef]
- Magin, C.M.; May, R.M.; Drinker, M.C.; Cuevas, K.H.; Brennan, A.B.; Reddy, S.T. Micropatterned protective membranes inhibit lens epithelial cell migration in posterior capsule opacification model. Transl. Vis. Sci. Technol. 2015, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Ball, P. Shark skin and other solutions. Nature 1999, 400, 507–509. [Google Scholar] [CrossRef]
- Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Schumacher, J.F.; Wilkerson, W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Brennan, A.B. Engineered antifouling microtopographies—Correlating wettability with cell attachment. Biofouling 2006, 22, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Scardino, A.J.; Zhang, H.; Cookson, D.J.; Lamb, R.N.; de Nys, R. The role of nano-roughness in antifouling. Biofouling 2009, 25, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.M.; Habimana, O.; Lawler, J.; O’reilly, B.; Daniels, S.; Casey, E.; Cowley, A. Cicada wing surface topography: An investigation into the bactericidal properties of nanostructural features. ACS Appl. Mater. Interfaces 2016, 8, 14966–14974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal effects of natural nanotopography of dragonfly wing on escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [Green Version]
- Minoura, K.; Yamada, M.; Mizoguchi, T.; Kaneko, T.; Nishiyama, K.; Ozminskyj, M.; Koshizuka, T.; Ikuo, W.; Tatsuo, S. Antibacterial effects of the artificial surface of nanoimprinted moth-eye film. PLoS ONE 2017, 12, e0185366. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopf, L.; Green, D.W. The nanotipped hairs of gecko skin and biotemplated replicas impair and/or kill pathogenic bacteria with high efficiency. Nanoscale 2016, 8, 18860–18869. [Google Scholar] [CrossRef]
- Diu, T.; Faruqui, N.; Sjöström, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-inspired cell-instructive nanopatterned arrays. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Dickson, M.N.; Liang, E.I.; Rodriguez, L.A.; Vollereaux, N.; Yee, A.F. Nanopatterned polymer surfaces with bactericidal properties. Biointerphases 2015, 10, 021010. [Google Scholar] [CrossRef] [Green Version]
- Hazell, G.; Fisher, L.E.; Murray, W.A.; Nobbs, A.H.; Su, B. Bioinspired bactericidal surfaces with polymer nanocone arrays. J. Colloid Interface Sci. 2018, 528, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Hazell, G.; May, P.W.; Taylor, P.; Nobbs, A.H.; Welch, C.C.; Su, B.B. Studies of black silicon and black diamond as materials for antibacterial surfaces. Biomater. Sci. 2018, 6, 1424–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Jain, S.; Chatterjee, K. Nanoscale topography on black titanium imparts multi-biofunctional properties for orthopedic applications. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linklater, D.P.; Nguyen, H.K.D.; Bhadra, C.M.; Juodkazis, S.; Ivanova, E.P. Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces. Nanotechnology 2017, 28, 245301. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.P.; Boshkovik, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.; Liu, J.; Guo, L.; Zhang, L.; Li, Q. Theoretical study on the bactericidal nature of nanopatterned surfaces. J. Theor. Biol. 2015, 385, 1–7. [Google Scholar] [CrossRef]
- Li, X. Bactericidal mechanism of nanopatterned surfaces. Phys. Chem. Chem. Phys. 2015, 18, 1311–1316. [Google Scholar] [CrossRef]
- Wu, S.; Zuber, F.; Maniura-Weber, K.; Brugger, J.; Ren, Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnol. 2018, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Watson, G.S.; Green, D.W.; Watson, J.A.; Zhou, Z.; Li, X.; Cheung, G.S.P.; Gellender, M. A simple model for binding and rupture of bacterial cells on nanopillar surfaces. Adv. Mater. Interfaces 2019, 6, 1801646. [Google Scholar] [CrossRef]
- Velic, A.; Tesfamichael, T.; Li, Z. Yarlagadda PKD V. Parametric study on nanopattern bactericidal activity. Procedia Manuf. 2019, 30, 514–521. [Google Scholar] [CrossRef]
- Ayazi, M.; Ebrahimi, N.G.; Nodoushan, E.J. Bacterial adhesion reduction on the surface with a simulated pattern: An insight into extrand model. Int. J. Adhes. Adhes. 2019, 88, 66–73. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Dutcher, J.R. Viscoelasticity of the bacterial cell envelope. Soft Matter 2011, 7, 4101–4110. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Li, X.; Chen, T. Enhancement and suppression effects of a nanopatterned surface on bacterial adhesion. Phys. Rev. E 2016, 93, 52419. [Google Scholar]
- Olivi, M.; Zanni, E.; De Bellis, G.; Talora, C.; Sarto, C.; Palleschi, M.S.; Flahaut, C.; Monthioux, E.; Papino, M.; Uccelletti, S. Inhibition of microbial growth by carbon nanotube networks. Nanoscale 2013, 5, 9023–9029. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.; Rao, K.K.; Suraishkumar, G.K. Reactive oxygen species induced by shear stress mediate cell death in Bacillus subtilis. Biotechnol. Bioeng. 2006, 94, 118–127. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone–implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Thompson, G.J.; Puleo, D.A. Effects of sublethal metal ion concentrations on osteogenic cells derived from bone marrow stromal cells. J. Appl. Biomater. 1995, 6, 249–258. [Google Scholar] [CrossRef]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7, S515–S527. [Google Scholar] [CrossRef]
- Karazisis, D.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Norlindh, B.; Johansson, A.; Rasmusson, L.; Thomsen, P.; Omar, O. The influence of controlled surface nanotopography on the early biological events of osseointegration. Acta Biomater. 2017, 53, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Karazisis, D.; Ballo, A.M.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Thomsen, P.; Omar, O. The role of well-defined nanotopography of titanium implants on osseointegration: Cellular and molecular events in vivo. Int. J. Nanomed. 2016, 11, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, T.; Dalby, M.J.; Hart, A.; Tare, R.; Oreffo, R.O.C.; Su, B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009, 5, 1433–1441. [Google Scholar] [CrossRef]
- Ahn, J.; Son, S.J.; Min, J. The control of cell adhesion on a PMMA polymer surface consisting of nanopillar arrays. J. Biotechnol. 2013, 164, 543–548. [Google Scholar] [CrossRef]
- Lim, J.Y.; Hansen, J.C.; Siedlecki, C.A.; Runt, J.; Donahue, H.J. Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. J. R. Soc. Interface 2005, 2, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.J.; Andar, A.; Nag, A.; Affrossman, S.; Tare, R.; McFarlane, S.; Oreffo, R.O.C. Genomic expression of mesenchymal stem cells to altered nanoscale topographies. J. R. Soc. Interface 2008, 5, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.J.; McCloy, D.; Robertson, M.; Agheli, H.; Sutherland, D.; Affrossman, S.; Oreffo, R.O.C. Osteoprogenitor response to semi-ordered and random nanotopographies. Biomaterials 2006, 27, 2980–2987. [Google Scholar] [CrossRef]
- Yu, P.; Zhu, X.; Wang, X.; Wang, S.; Li, W.; Tan, G.; Zhang, Y.; Ning, C. Periodic nanoneedle and buffer zones constructed on a titanium surface promote osteogenic differentiation and bone calcification in vivo. Adv. Healthc. Mater. 2016, 5, 364–372. [Google Scholar] [CrossRef]
- McNamara, L.E.; Sjöström, T.; Burgess, K.E.V.; Kim, J.J.W.; Liu, E.; Gordonov, S.; Moghe, P.V.; Meek, R.M.D.; Oreffo, R.O.C.; Su, B. Skeletal stem cell physiology on functionally distinct titania nanotopographies. Biomaterials 2011, 32, 7403–7410. [Google Scholar] [CrossRef]
- Silverwood, R.K.; Fairhurst, P.G.; Sjöström, T.; Welsh, F.; Sun, Y.; Li, G.; Yu, B.; Young, P.S.; Su, B.; Meek, R.M.D.; et al. Analysis of osteoclastogenesis/osteoblastogenesis on nanotopographical titania surfaces. Adv. Healthc. Mater. 2016, 5, 947–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papi, P.; Raco, A.; Pranno, N.; Di Murro, B.; Passarelli, P.C.; D’Addona, A.; Pompa, G.; Barbieri, M. Salivary levels of titanium, nickel, vanadium, and arsenic in patients treated with dental implants: A case-control study. J. Clin. Med. 2020, 9, 1264. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.D.; Aten, K.; Krause, A.J.; Metzger, M.L.; Holloway, S.S. Creating a university technology commercialisation programme: Confronting conflicts between learning, discovery and commercialisation goals. Int. J. Entrep. Innov. Manag. 2011, 13, 179–197. [Google Scholar] [CrossRef]
- Busscher, H.J.; Van Der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; Van Den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Moure, J.S. Lost in translation: The gap in scientific advancements and clinical application. Front. Bioeng. Biotechnol. 2016, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.L.; Tan, Y.N.; Mohamed, A.R. A review on the formation of titania nanotube photocatalysts by hydrothermal treatment. J. Environ. Manag. 2011, 92, 1669–1680. [Google Scholar] [CrossRef]
- Lee, J.; Kang, B.S.; Hicks, B.; Chancellor, T.F., Jr.; Chu, B.H.; Wang, H.T.; Keselowsky, B.G.; Ren, F.; Lele, T.P. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials 2008, 29, 3743–3749. [Google Scholar] [CrossRef]
- Shim, J.B.; Chang, H.; Kim, S.-O. Rapid hydrothermal synthesis of zinc oxide nanowires by annealing methods on seed layers. J. Nanomater. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Joshi, U.A.; Yoon, S.; Baik, S.; Lee, J.S. Surfactant-free hydrothermal synthesis of highly tetragonal barium titanate nanowires: A structural investigation. J. Phys. Chem. B 2006, 110, 12249–12256. [Google Scholar] [CrossRef]
- Bao, N.; Shen, L.; Srinivasan, G.; Yanagisawa, K.; Gupta, A. Shape-controlled monocrystalline ferroelectric barium titanate nanostructures: From nanotubes and nanowires to ordered nanostructures. J. Phys. Chem. C 2008, 112, 8634–8642. [Google Scholar] [CrossRef]
- Joshi, U.A.; Lee, J.S. Template-free hydrothermal synthesis of single-crystalline barium titanate and strontium titanate nanowires. Small 2005, 1, 1172–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Xu, J.; Yang, D. Hydrothermal synthesis of flower-like SrCO3 nanostructures. Mater. Lett. 2005, 59, 420–422. [Google Scholar] [CrossRef]
- Kim, T.-G.; Park, B. Synthesis and growth mechanisms of one-dimensional strontium hydroxyapatite nanostructures. Inorg. Chem. 2005, 44, 9895–9901. [Google Scholar] [CrossRef] [PubMed]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018, 9, 2041731418790694. [Google Scholar] [CrossRef] [PubMed]
- Goriainov, V.; Hulsart-Billstrom, G.; Sjostrom, T.; Dunlop, D.G.; Su, B.; Oreffo, R.O.C. Harnessing nanotopography to enhance osseointegration of clinical orthopedic titanium implants—An in vitro and in vivo analysis. Front. Bioeng. Biotechnol. 2018, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Su, B.; Chinnaraj, S.; Jana, S.; Bowen, L.; Charlton, S.; Duan, P.; Jakubovics, N.S.; Chen, J. Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.D.; Su, B.; Dalby, M.J. Osteogenic and bactericidal surfaces from hydrothermal titania nanowires on titanium substrates. Sci. Rep. 2016, 6, 36857. [Google Scholar] [CrossRef]
- Sjöström, T.; Nobbs, A.H.; Su, B. Bactericidal nanospike surfaces via thermal oxidation of Ti alloy substrates. Mater. Lett. 2016, 167, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Boercker, J.E.; Aydil, E.S. Oriented single crystalline titanium dioxide nanowires. Nanotechnology 2008, 19, 505604. [Google Scholar] [CrossRef]
- Yada, M.; Inoue, Y.; Uota, M.; Torikai, T.; Watari, T.; Noda, I.; Hotokebuchi, T. Plate, wire, mesh, microsphere, and microtube composed of sodium titanate nanotubes on a titanium metal template. Langmuir 2007, 23, 2815–2823. [Google Scholar] [CrossRef]
- Umehara, H.; Kobatake, R.; Oki, Y.; Makihara, Y.; Kubo, T.; Tsuga, K. Histological and bone morphometric evaluation of osseointegration aspects by alkali hydrothermally-treated implants. Appl. Sci. 2018, 8, 635. [Google Scholar] [CrossRef] [Green Version]
- De Viteri, V.S.; Fuentes, E. Titanium and titanium alloys as biomaterials. Tribol.-Fundam. Adv. 2013, 155–181. [Google Scholar]
- Jenkins, J.J. An Alternative Approach to Combat Antimicrobial Resistant Infections of Medical Implants And Devices. Ph.D. Thesis, University of Bristol, Bristol, UK, November 2019. [Google Scholar]
- Wang, G.; Li, J.; Lv, K.; Zhang, W.; Ding, X.; Yang, G.; Liu, X.; Jiang, X. Surface thermal oxidation on titanium implants to enhance osteogenic activity and in vivo osseointegration. Sci. Rep. 2016, 6, 31769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbie, K.; Sit, J.C.; Brett, M.J. Advanced techniques for glancing angle deposition. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. Process. Meas. Phenom. 1998, 16, 1115–1122. [Google Scholar] [CrossRef]
- Taschuk, M.T.; Hawkeye, M.M.; Brett, M.J. Glancing angle deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 621–678. [Google Scholar]
- Motemani, Y.; Greulich, C.; Khare, C.; Lopian, M.; Buenconsejo, P.J.S.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Adherence of human mesenchymal stem cells on Ti and TiO2 nano-columnar surfaces fabricated by glancing angle sputter deposition. Appl. Surf. Sci. 2014, 292, 626–631. [Google Scholar] [CrossRef]
- Sengstock, C.; Lopian, M.; Motemani, Y.; Borgmann, A.; Khare, C.; Buenconsejo, P.J.S.; Schildhauer, T.A.; Ludwig, A.; Köller, M. Structure-related antibacterial activity of a titanium nanostructured surface fabricated by glancing angle sputter deposition. Nanotechnology 2014, 25, 195101. [Google Scholar] [CrossRef]
- Alvarez, R.; Muñoz-Piña, S.; González, M.U.; Izquierdo-Barba, I.; Fernández-Martínez, I.; Rico, V.; Arcos, D.; García-Valenzuela, A.; Palmero, A.; Vallet-Regi, M.; et al. Antibacterial nanostructured ti coatings by magnetron sputtering: From laboratory scales to industrial reactors. Nanomaterials 2019, 9, 1217. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, N.; Sengstock, C.; Mai, V.; Schildhauer, T.A.; Köller, M.; Ludwig, A. Glancing-angle deposition of nanostructures on an implant material surface. Nanomaterials 2019, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- Ganjian, M.; Modaresifar, K.; Zhang, H.; Hagedoorn, P.-L.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Reactive ion etching for fabrication of biofunctional titanium nanostructures. Sci. Rep. 2019, 9, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.P.; Laskar, M.R.; Azizur Rahman, A.; Gokhale, M.R.; Bhattacharya, A. Inductively coupled plasma–reactive ion etching of c-and a-plane AlGaN over the entire Al composition range: Effect of BCl3 pretreatment in Cl2/Ar plasma chemistry. J. Vac. Sci. Technol. A Vac. Surf. Films 2013, 31, 61305. [Google Scholar] [CrossRef]
- Pham, V.T.H.; Truong, V.K.; Orlowska, A.; Ghanaati, S.; Barbeck, M.; Booms, P.; Fulcher, A.J.; Bhadra, C.M.; Buividas, R.; Baulin, V. “Race for the surface”: Eukaryotic cells can win. ACS Appl. Mater. Interfaces 2016, 8, 22025–22031. [Google Scholar] [CrossRef] [Green Version]
- Hoyos-Nogués, M.; Velasco, F.; Ginebra, M.-P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: The blending of cell integration and bacterial inhibition properties on the surface of biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 21618–21630. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A. Nanostructures as promising tools for delivery of antimicrobial peptides. Mini Rev. Med. Chem. 2012, 12, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Böge, C.; Janissen, R.; Rohrbeck, D.; Hülsen, T.; Lensing-Höhn, S.; Krauspe, R.; Herten, M. Osteoblastic potency of bone marrow cells cultivated on functionalized biometals with cyclic RGD-peptide. J. Biomed. Mater. Res. Part A 2013, 101, 2905–2914. [Google Scholar] [CrossRef]
- Fraioli, R.; Tsimbouri, P.M.; Fisher, L.E.; Nobbs, A.H.; Su, B.; Neubauer, S.; Rechenmacher, F.; Kessler, H.; Ginebra, M.P.; Dalby, M.J.; et al. Towards the cell-instructive bactericidal substrate: Exploring the combination of nanotopographical features and integrin selective synthetic ligands. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.-F.; Vashist, S.K.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.-S. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem. Res. Toxicol. 2010, 23, 1131–1147. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishak, M.I.; Liu, X.; Jenkins, J.; Nobbs, A.H.; Su, B. Protruding Nanostructured Surfaces for Antimicrobial and Osteogenic Titanium Implants. Coatings 2020, 10, 756. https://doi.org/10.3390/coatings10080756
Ishak MI, Liu X, Jenkins J, Nobbs AH, Su B. Protruding Nanostructured Surfaces for Antimicrobial and Osteogenic Titanium Implants. Coatings. 2020; 10(8):756. https://doi.org/10.3390/coatings10080756
Chicago/Turabian StyleIshak, Mohd I., Xiayi Liu, Joshua Jenkins, Angela H. Nobbs, and Bo Su. 2020. "Protruding Nanostructured Surfaces for Antimicrobial and Osteogenic Titanium Implants" Coatings 10, no. 8: 756. https://doi.org/10.3390/coatings10080756