Individual and Combined Coatings of Chitosan and Carnauba Wax with Oregano Essential Oil to Avoid Water Loss and Microbial Decay of Fresh Cucumber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Film Formulation
2.4. Characterization of the Formulated Films
2.4.1. Thickness and Water Vapor Transmission (WVT)
2.4.2. In Vitro Antimicrobial Capacity of the Formulated Films
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Effect of the Formulated Coatings on Water Loss and Microbial Decay of Fresh Cucumbers
2.5.1. Coating Application
2.5.2. Fruit Weight Loss
2.5.3. Changes of Microbial Load of the Coated Fruit
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Formulated Films
3.1.1. FTIR Spectra
3.1.2. Thickness and WVT
3.1.3. In Vitro Antimicrobial Activity of Films
3.2. Postharvest Changes of Coated Cucumbers
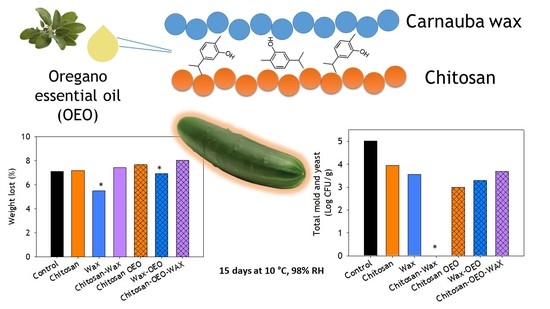
3.2.1. Coating Influence on Postharvest Weight Loss
3.2.2. The Microbial Load of Coated Cucumbers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USDA. Food Data Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/168409/nutrients (accessed on 28 April 2020).
- Barraza-Álvarez, F.V. Calidad morfológica y fisiológica de pepinos cultivados en diferentes concentraciones nutrimentales. Revista Colombiana de Ciencias Horticolas 2015, 9, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Olawuyi, I.F.; Lee, W. Influence of chitosan coating and packaging materials on the quality characteristics of fresh-cut cucumber. Korean J Food Preserv 2019, 26, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.P. Bacterial Diseases and Their Management. In In Sustainable Crop Protection under Protected Cultivation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 153–159. [Google Scholar]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N.J.I.J.o.B.M. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G.J.E.J.o.F. Agriculture. Effects of edible coatings on quality maintenance of fresh-cut nectarines. Emir. J. Food Agric. 2016, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Moreira, M.d.R.; Roura, S.I.; Ponce, A. Effectiveness of chitosan edible coatings to improve microbiological and sensory quality of fresh cut broccoli. LWT Food Sci. Technol. 2011, 44, 2335–2341. [Google Scholar] [CrossRef]
- Pavinatto, A.; De Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Athmaselvi, K.; Sumitha, P.; Revathy, B.J.I.A. Development of Aloe vera based edible coating for tomato. Int. Agrophys. 2013, 27, 369–375. [Google Scholar] [CrossRef]
- Poverenov, E.; Zaitsev, Y.; Arnon, H.; Granit, R.; Alkalai-Tuvia, S.; Perzelan, Y.; Weinberg, T.; Fallik, E. Effects of a composite chitosan–gelatin edible coating on postharvest quality and storability of red bell peppers. Postharvest. Biol. Technol. 2014, 96, 106–109. [Google Scholar] [CrossRef]
- De Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef]
- Won, M.Y.; Min, S.C. Coating Satsuma mandarin using grapefruit seed extract–incorporated carnauba wax for its preservation. Food Sci. Biotechnol. 2018, 27, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, E.; Nasiri, J.; Malidarreh, T.R.; Kalantari, S.; Naghavi, M.R.; Safari, M. Performance of carnauba wax-nanoclay emulsion coatings on postharvest quality of ‘Valencia’ orange fruit. Sci. Hortic 2018, 240, 170–178. [Google Scholar] [CrossRef]
- Rodríguez-Rivera, R.; Herrera-González, J.; Mercado-Silva, E.; Vázquez-Barrios, M.; Rivera-Pastrana, D. Natural coatings and essential oils effects on postharvest quality and antioxidant system of organic avocado (Persea americana Mill ’Hass’). In Proceedings of XXX International Horticultural Congress IHC2018: International Symposium on Strategies and Technologies to Maintain Quality 1275, Istanbul, Turkey, 16 August 2018; pp. 147–154. [Google Scholar]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Sharma, L.; Maity, T. Chapter 34—Enrichment of edible coatings and films with plant extracts or essential oils for the preservation of fruits and vegetables. In Biopolymer-Based Formulations; Pal, K., Banerjee, I., Sarkar, P., Kim, D., Deng, W.-P., Dubey, N.K., Majumder, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 859–880. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Cruz-Valenzuela, M.R.; Silva-Espinoza, B.A.; Gonzalez-Aguilar, G.A.; Moctezuma, E.; Gutierrez-Pacheco, M.M.; Tapia-Rodriguez, M.R.; Ortega-Ramirez, L.A.; Ayala-Zavala, J.F. Oregano (Lippia graveolens) essential oil added within pectin edible coatings prevents fungal decay and increases the antioxidant capacity of treated tomatoes. J. Sci. Food Agric. 2016, 96, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, S.; Pu, T.; Fan, L.; Su, F.; Ye, M. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat. Prod. Res. 2019, 33, 1423–1430. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. 2019, 1–12. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest. Biol. Technol. 2015, 110, 203–213. [Google Scholar] [CrossRef]
- ORE. ORE-Orégano Orgánico Lippia Graveolens. Available online: http://www.oreganoorganico.com/ (accessed on 15 April 2020).
- ASTM International. Standard Test Methods for Water Vapor Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Clinical and Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing: Eleventh Informational; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2001. [Google Scholar]
- BAM-FDA. Bacteriological Analytical Manual. Available online: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam#intro (accessed on 10 January 2020).
- El Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Ochoa, T.A.; Almendárez, B.E.G.; Reyes, A.A.; Pastrana, D.M.R.; López, G.F.G.; Belloso, O.M.; Regalado-González, C. Design and characterization of corn starch edible films including beeswax and natural antimicrobials. Food Bioprocess Technol. 2017, 10, 103–114. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and antifungal activity of pomegranate peel extract and its use in polysaccharide-based edible coatings to extend the shelf-life of capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327. [Google Scholar] [CrossRef]
- Santos, F.K.G.d.; Silva, K.N.d.O.; Xavier, T.D.N.; Leite, R.H.d.L.; Aroucha, E.M.M. Effect of the addition of carnauba wax on physicochemical properties of chitosan films. Mater. Res. 2017, 20, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Haq, M.A.; Hasnain, A.; Jafri, F.A.; Akbar, M.F.; Khan, A. Characterization of edible gum cordia film: Effects of beeswax. LWT Food Sci. Technol. 2016, 68, 674–680. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Kong, L.; Hou, H.J.C.P. Effects of preparation conditions on the properties of agar/maltodextrin-beeswax pseudo-bilayer films. Carbohydr. Polym. 2020, 236, 116029. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Morsi, R.E.; Fathy, M. Chapter 44 - Chitosan-Oregano Essential Oil Blends Use as Antimicrobial Packaging Material. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 539–551. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Maté, J.I.; Gardrat, C.; Coma, V. Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll. 2015, 51, 60–68. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with Thymus moroderi or Thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Bosquez-Molina, E.; Guerrero-Legarreta, I.; Vernon-Carter, E. Moisture barrier properties and morphology of mesquite gum–candelilla wax based edible emulsion coatings. Food Res. Int. 2003, 36, 885–893. [Google Scholar] [CrossRef]
- Endlein, E.; Peleikis, K.H. Natural Waxes―Properties, Compositions and Applications. SÖFW-Journal 2011, 137. [Google Scholar]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Miranda, M.; Gozalbo, A.M.; Sun, X.; Plotto, A.; Bai, J.; de Assis, O.; Ferreira, M.; Baldwin, E. Effect of mono and bilayers of carnauba wax based nano-emulsion and HPMC coatings on popst-harvest quality of’redtainung’papaya. Proceedings of Embrapa Instrumentação-Artigo em Anais de Congresso (ALICE), São Paulo, Brazil, 3–5 December 2019. [Google Scholar]
- Chiabrando, V.; Giacalone, G. Effect of chitosan and sodium alginate edible coatings on the postharvest quality of fresh-cut nectarines during storage. Int. J. Trop. Subtrop. Hortic. 2016, 71, 79–85. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Scientia Hortic 2020, 259, 108656. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.d.R. Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT Food Sci. Technol. 2013, 50, 78–87. [Google Scholar] [CrossRef]
- Walker, G.M.; White, N.A. Introduction to fungal physiology. In Fungi: Biology and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–35. [Google Scholar] [CrossRef] [Green Version]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT Food Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Kanetis, L.; Exarchou, V.; Charalambous, Z.; Goulas, V. Edible coating composed of chitosan and Salvia fruticosa Mill. extract for the control of grey mould of table grapes. J. Sci. Food Agric. 2017, 97, 452–460. [Google Scholar] [CrossRef]
- Kumar, V.; Sangeetha, K.; Ajitha, P.; Aisverya, S.; Sashikala, S.; Sudha, P.J.H.o.B.A.; Applications, M. Chitin and Chitosan: The Defense Booster in Agricultural Field. In In Handbook of Biopolymers: Advances and Multifaceted Application; Vijayalakshmi, K.S.K., Ajitha, P., Aisverya, S., Sashikala, S., Sudha, P.N., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2018; p. 93. [Google Scholar]






| Parameter | Mean ± Standard Deviation |
|---|---|
| Firmness (N) | 48.80 ± 1.89 |
| CO2 production rate (mL CO2/kg·h) | 1.10 ± 0.65 |
| Color | – |
| Lightness | 29.21 ± 1.01 |
| Chroma | 59.50 ± 13.19 |
| Hue | 132.76 ± 0.54 |
| Film | Thickness (mm) * | WVT (g/m² h) ** |
|---|---|---|
| Chitosan | 0.025 ± 0.007 a*** | 0.257 ± 0.013 a |
| Chitosan-OEO | 0.022 ± 0.002 a | 0.241 ± 0.019 a |
| Chitosan-Wax | 0.027 ± 0.005 a | 0.167 ± 0.012 b |
| Chitosan-OEO-Wax | 0.037 ± 0.004 b | 0.141 ± 0.008 b |
| Treatment | Weight Loss at Day 15 (%) |
|---|---|
| Control | 7.11 ± 0.20 b* |
| Chitosan | 7.18 ± 0.50 b |
| Wax | 5.48 ± 0.50 a |
| Chitosan-wax | 7.42 ± 0.21 b |
| Chitosan-OEO | 7.66 ± 0.29 b |
| Wax-OEO | 6.93 ± 0.10 a |
| Chitosan-OEO-wax | 8.03 ± 2.01 b |
| Storage (Days) | Log CFU·g−1 * | ||||||
|---|---|---|---|---|---|---|---|
| Control | Chitosan | Wax | Chitosan-Carnauba Wax | Chitosan-OEO | Wax-OEO | Chitosan- OEO-Wax | |
| 0 | 6.70 a* | 3.81 b | 4.44 b | 4.62 b | 3.76 b | 4.94 b | 4.80 b |
| 3 | 6.0 c | 4.80 a | 5.45 b | 4.90 ab | 5.20 ab | 5.36 ab | 4.96 ab |
| 6 | 5.90 b | 4.80 a | 6.97 c | 5.0 a | 4.85 a | 6.98 c | 5.09 a |
| 9 | 5.23 bc | 4.70 a | 5.54 c | 4.85 ab | 5.51 c | 5.34 c | 5.11 abc |
| 12 | 6.19 c | 5.22 a | 5.39 ab | 5.99 c | 5.73 bc | 6.30 c | 5.80 bc |
| 15 | 7.37 d | 5.78 a | 6.90 c | 6.68 bc | 6.39 b | 6.89 c | 6.56 b |
| Storage (Days) | Log CFU·g−1 * | ||||||
|---|---|---|---|---|---|---|---|
| Control | Chitosan | Wax | Chitosan-Wax | Chitosan-OEO | Wax-OEO | Chitosan–OEO-Wax | |
| 0 | 3.58 c* | 2.14 ab | 2.30 ab | 2.18 ab | 1.82 ab | 2.72 bc | 1.87 a |
| 3 | 5.07 c | 3.72 a | 5.22 d | 4.12 ab | 4.10 ab | 4.69 bcd | 4.27 abc |
| 6 | 4.30 b | 3.0 a | 3.53 a | – | – | 3.37 a | 3 a |
| 9 | 4.25 b | 3.20 a | – | – | 4.13 b | 3.07 a | – |
| 12 | 4.98 c | 4.12 b | 3 a | 3.0 a | 3 a | 4.05 b | – |
| 15 | 5.02 c | 3.94 b | 3.55 ab | – | 3 a | 3.30 ab | 3.69 ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Pacheco, M.M.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Lizardi-Mendoza, J.; Miranda, R.; Ayala-Zavala, J.F. Individual and Combined Coatings of Chitosan and Carnauba Wax with Oregano Essential Oil to Avoid Water Loss and Microbial Decay of Fresh Cucumber. Coatings 2020, 10, 614. https://doi.org/10.3390/coatings10070614
Gutiérrez-Pacheco MM, Ortega-Ramírez LA, Silva-Espinoza BA, Cruz-Valenzuela MR, González-Aguilar GA, Lizardi-Mendoza J, Miranda R, Ayala-Zavala JF. Individual and Combined Coatings of Chitosan and Carnauba Wax with Oregano Essential Oil to Avoid Water Loss and Microbial Decay of Fresh Cucumber. Coatings. 2020; 10(7):614. https://doi.org/10.3390/coatings10070614
Chicago/Turabian StyleGutiérrez-Pacheco, María Melissa, Luis Alberto Ortega-Ramírez, Brenda Adriana Silva-Espinoza, Manuel Reynaldo Cruz-Valenzuela, Gustavo Adolfo González-Aguilar, Jaime Lizardi-Mendoza, Raquel Miranda, and Jesús Fernando Ayala-Zavala. 2020. "Individual and Combined Coatings of Chitosan and Carnauba Wax with Oregano Essential Oil to Avoid Water Loss and Microbial Decay of Fresh Cucumber" Coatings 10, no. 7: 614. https://doi.org/10.3390/coatings10070614