Thermal Stability of Rare Earth-PYSZ Thermal Barrier Coating with High-Resolution Transmission Electron Microscopy
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Isothermal Test
2.2. Thermal Cycle Test
2.3. Characterization
2.4. Sample Preparation for SEM and HRTEM
2.5. Thermal Stability and Strain Measurement with HRTEM
3. Results
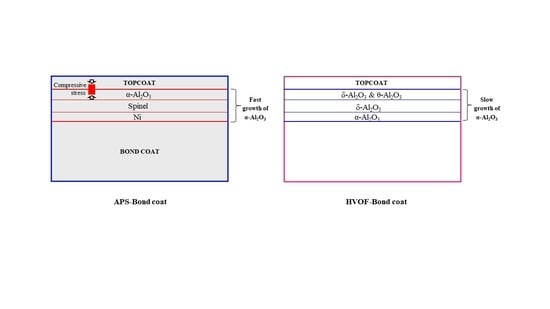
3.1. Microstructure and TGO Growth
3.2. Thermal Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kano, K.; Matsuzaki, H.; Aoyama, K.; Aoki, S.; Mandai, S. Development Study of 1500 °C Class High Temperature Gas Turbine. In Proceedings of the Turbo Expo: Power for Land, Sea, and Air, ASME 1991 International Gas Turbine and Aeroengine Congress and Exposition, Orlando, FL, USA, 3–6 June 1991; American Society of Mechanical Engineers: New York, NY, USA, 1991. [Google Scholar]
- Ishikawa, M.; Terauchi, M.; Komori, T.; Yasuraoka, J. Development of High Efficiency Gas Turbine Combined Cycle Power Plant. Mitsubishi Heavy Ind. Tech. Rev. 2008, 45, 15–17. [Google Scholar]
- Manap, A.; Okabe, T.; Ogawa, K.; Mahalingam, S.; Abdullah, H. Experimental and Smoothed Particle Hydrodynamics Analysis of Interfacial Bonding Between Aluminum Powder Particles and Aluminum Substrate by Cold Spray Technique. Int. J. Adv. Manuf. Technol. 2019, 103, 4519–4527. [Google Scholar] [CrossRef]
- Xia, J.; Yang, L.; Wu, R.; Zhou, Y.; Zhang, L.; Huo, K.L.; Gan, M. Degradation Mechanisms of Air Plasma Sprayed Free-standing Yttria-stabilized Zirconia Thermal Barrier Coatings Exposed to Volcanic Ash. Appl. Surf. Sci. 2019, 481, 860–871. [Google Scholar] [CrossRef]
- Mahalingam, S.; Mohd Yunus, S.; Manap, A.; Afandi, N.M.; Zainuddin, R.A.; Kadir, N.F. Crack Propagation and Effect of Mixed Oxides on TGO Growth in Thick La-Gd-YSZ Thermal Barrier Coating. Coatings 2019, 9, 719. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, T.; Li, C.; Zheng, Z.; Li, Q. Microstructural evolution and growth kinetics of thermally grown oxides in plasma sprayed thermal barrier coatings. Prog. Nat. Sci. 2016, 26, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Hayase, T.; Waki, H.; Adachi, K. Residual Stress Change in Thermal Barrier Coating Due to Thermal Exposure Evaluated by Curvature Method. J. Therm. Spray Tech. 2020, 29, 1300–1312. [Google Scholar] [CrossRef]
- Li, L.; Hitchman, N.; Knapp, J. Failure of Thermal Barrier Coatings Subjected to CMAS Attack. J. Therm. Spray Tech. 2010, 19, 148–155. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Hussain, M.S. Improved Thermally Grown Oxide Scale in Air Plasma Sprayed NiCrAlY/Nano-YSZ. Coatings. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yajid, M.A.M.; Yusof, N.M.; Hussain, M. Formation of a dense and continuous Al2O3 layer in nano thermal barrier coating systems for the suppression of spinel growth on the Al2O3 oxide scale during oxidation. J. Alloys Compd. 2013, 571, 205–220. [Google Scholar] [CrossRef]
- Wu, R.T.; Osawa, M.; Yokokawa, T.; Kawagishi, K.; Harada, H. Degradation Mechanisms of an Advanced Jet Engine Service-Retired TBC Component. J. Solid Mech. 2010, 4, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.V. Use of Thermally Grown Oxide Stress Measurements to Predict Remaining Life of Thermal Barrier Coatings under Realistic Turbine Engine Conditions. Master’s Thesis, University of Connecticut Graduate School, Storrs, CT, USA, 12 December 2014. [Google Scholar]
- Stiger, M.; Yanar, N.; Jackson, R.; Laney, S.; Pettit, F.; Meier, G.; Gandhi, A.; Levi, C.G. Development of Intermixed Zones of Alumina/Zirconia in Thermal Barrier Coating Systems. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2007, 38, 848–857. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Ando, Y.; Kurokawa, K.; Hejwowski, T. Microstructure and thermal behavior of plasma sprayed zirconia/alumina composite coating. J. Nanosci. Nanotechnol. 2011, 11, 8853–8858. [Google Scholar] [CrossRef] [PubMed]
- Huo, P.; Song, W.; Zhou, X.; Zhang, H.; Jiang, J.; Dong, S.; Cao, X.; Dingwell, D.B. Microstructures and Properties of Sm2(Zr0.7Ce0.3)2O7/8YSZ Double-ceramic-layer Thermal Barrier Coatings Deposited by Atmospheric Plasma Spraying. J. Therm. Spray Tech. 2019, 28, 986–999. [Google Scholar] [CrossRef]
- Wang, H.; Muralidharan, G.; Leonard, D.N.; Haynes, J.A.; Porter, W.D.; England, R.D.; Hays, M.; Dwivedi, G.; Sampath, S. Microstructural Analysis and Transport Properties of Thermally Sprayed Multiple-Layer Ceramic Coatings. J. Therm. Spray Tech. 2018, 27, 371–378. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, H.; Liu, Z.; Shi, W. Sampling Moiré Method and Its Application to Determine Modulus of Thermal Barrier Coatings under Scanning Electron Microscope. Opt. Laser Eng. 2018, 107, 315–324. [Google Scholar] [CrossRef]
- Mutter, M.; Mauer, G.; Mücke, R.; Guillon, O.; Vaßen, R. Systematic İnvestigation on the İnfluence of Spray Parameters on the Mechanical Properties of Atmospheric Plasma-sprayed YSZ Coatings. J. Therm. Spray Tech. 2018, 27, 566–580. [Google Scholar] [CrossRef]
- Madsen, J.; Liu, P.; Wagner, J.B.; Hansen, T.W.; Schiøtz, J. Accuracy of Surface Strain Measurements from Transmission Electron Microscopy Images of Nanoparticles. Adv. Struct. Chem. Imag. 2017, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Béché, A.; Rouvière, J.L.; Barnes, J.P.; Cooper, D. Strain Measurement at The Nanoscale: Comparison Between Convergent Beam Electron Diffraction, Nano-beam Electron diffraction, High Resolution Imaging and Dark Field Electron Holography. Ultramicroscopy 2013, 131, 10–23. [Google Scholar] [CrossRef]
- Hÿtch, M.J.; Minor, A.M. Observing and Measuring Strain in Nanostructures and Devices with Transmission Electron Microscopy. MRS Bull. 2014, 39, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Vassen, R.; Cao, X.; Tietz, F.; Basu, D.; Stöver, D. Zirconates as New Materials for Thermal Barrier Coatings. J. Am. Ceram. Soc. 2000, 83, 2023–2028. [Google Scholar] [CrossRef]
- Ma, X.; Rivellini, K.; Ruggiero, P.; Wildridge, G. Toward Durable Thermal Barrier Coating with Composite Phases and Low Thermal Conductivity. J. Therm. Spray Tech. 2020, 29, 423–432. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, X.; Lin, C.; Jiang, C.; Zheng, W.; Chang, C.; Zeng, Y. Thermal Properties and Microstructures Analysis of YSZ and YSZ-Al2O3 Thermal Barrier Coatings. J. Therm. Spray Tech. 2020, 29, 574–581. [Google Scholar] [CrossRef]
- Mauer, G.; Du, L.; Vaßen, R. Atmospheric Plasma Spraying of Single Phase Lanthanum Zirconate Thermal Barrier Coatings with Optimized Porosity. Coatings 2016, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Krämer, S.; Yang, J.; Levi, C.G. Infiltration-inhibiting Reaction of Gadolinium Zirconate Thermal Barrier Coatings with CMAS Melts. J. Am. Ceram. Soc. 2008, 91, 576–583. [Google Scholar] [CrossRef]
- Hospach, A.; Mauer, G.; Vaßen, R.; Stöver, D. Columnar-structured Thermal Barrier Coatings (TBCs) by Thin Film Low-pressure Plasma spraying (LPPS-TF). J. Therm. Spray Tech. 2011, 20, 116–120. [Google Scholar] [CrossRef]
- Lu, Z.; Lyu, G.; Gulhane, A.; Park, H.-M.; Kim, J.S.; Jung, Y.-G.; Zhang, J. Experimental and Modeling Studies of Bond Coat Species Effect on Microstructure Evolution in EB-PVD Thermal Barrier Coatings in Cyclic Thermal Environments. Coatings 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Lance, M.J.; Thiesing, B.P.; Haynes, J.A.; Parish, C.M. The Effect of HVOF Bond Coating with APS Flash Coating on TBC Performance. Oxid. Met. 2019, 91, 5–6. [Google Scholar] [CrossRef]
- Wu, X.J.; Chen, W.R.; Patnaik, P.C. The Growth and Influence of Thermally Grown oxide in A Thermal Barrier Coating. In Proceedings of the Thermal Spray 2007: Global Coating Solutions, Beijing, China, 14–16 May 2007; Marple, B.R., Hyland, M.M., Lau, Y.-C., Li, C.-J., Lima, R.S., Montavon, G., Eds.; ASM Thermal Spray Society, ASM International: Novelty, OH, USA, 2007; pp. 446–451. [Google Scholar]
- Brinkman, H.W.; Briels, W.J.; Verweij, H. Molecular Dynamics Simulations of Yttrium-stabilized Zirconia. Chem. Phys. Lett. 1995, 247, 386–390. [Google Scholar] [CrossRef]
- Bengtsson, P.; Ericsson, T.; Wigren, J. Thermal Shock Testing of Burner Cans Coated with a Thick Thermal Barrier Coating. J. Therm. Spray Technol. 1998, 7, 340–348. [Google Scholar] [CrossRef]
- Mohd Zulkifli, S.; Mat Yajid, M.A.; Idris, M.H.; Daroonparvar, M.; Hamdan, H. TGO Formation with NiCoCrAlYTa Bond Coat Deposition using APS and HVOF Method. Adv. Mat. Res. 2015, 1125, 18–22. [Google Scholar] [CrossRef]
- Vernhes, L.; Bekins, C.; Lourdel, N.; Poirier, D.; Lima, R.; Li, D.; Klemberg-Sapieha, J. Nanostructured and Conventional Cr2O3, TiO2, and TiO2-Cr2O3 Thermal-Sprayed Coatings for Metal-Seated Ball Valve Applications in Hydrometallurgy. J. Therm. Spray Technol. 2016, 25, 1068–1078. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Zheng, S.J.; Zhu, Y.L.; Wei, H.; Ma, X.L. Microstructural Evolution at Interfaces of Thermal Barrier Coatings During Isothermal Oxidation. J. Eur. Ceram. Soc. 2016, 36, 1765–1774. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Yajid, M.A.; Yusof, N.M.; Farahany, S.; Hussain, M.S.; Bakhsheshi-Rad, H.R.; Valefi, Z.; Abdolahi, A. Improvement of Thermally Grown Oxide Layer in Thermal Barrier Coating Systems with Nano Alumina as Third Layer. T. Nanferr. Metal Soc. 2013, 23, 1322–1333. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Hussain, M.S.; Mat Yajid, M.A. The Role of Formation of Continues Thermally Grown Oxide Layer on the Nanostructured NiCrAlY Bond Coat During Thermal Exposure in Air. Appl. Surf. Sci. 2012, 261, 287–297. [Google Scholar] [CrossRef]
- Fujita, M.; Inukai, K.; Sakida, S.; Nanba, T.; Ommyoji, J.A. Yamaguchi, and Y. Miura, Sintering of Al2O3-Cr2O3 Powder Prepared by Sol-gel Process. J. Soc. Mater. Sci. Jpn. 2007, 56, 526–530. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Clarke, D.R.; Wang, F. Transient-alumina Transformations during the Oxidation of Magnetron-sputtered CoCrAl Nanocrystalline Coatings. Oxid. Met. 2003, 60, 225–240. [Google Scholar] [CrossRef]
- Liang, G.Y.; Zhu, C.; Wu, X.Y.; Wu, Y. The Formation Model of Ni-Cr Oxides on NiCoCrAlY-Sprayed Coating. Appl. Surf. Sci. 2011, 257, 6468–6473. [Google Scholar] [CrossRef]
- Kolarik, V.; Juez-Lorenzo, M.; Fietzek, H. Oxidation of Micro-Sized Spherical Aluminum Particles. Mater. Sci. Forum 2011, 696, 290–295. [Google Scholar] [CrossRef]
- Manap, A.; Nakano, A.; Ogawa, K. The Protectiveness of Thermally Grown Oxides on Cold Sprayed CoNiCrAlY Bond Coat in Thermal Barrier Coating. J. Therm. Spray Technol. 2012, 21, 586–596. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Sloof, W.G. Microstructural Evolution of a MCrAlY Coating upon Isothermal Annealing. Mater. Charact. 2008, 59, 1697–1704. [Google Scholar] [CrossRef]
- Teixeira, V.; Andritschky, M.; Fischer, W.; Buchkremer, H.P.; Stöver, D. Effects of Deposition Temperature and Thermal Cycling on Residual Stress State in Zirconia-based Thermal Barrier Coatings. Surf. Coat. Technol. 1999, 120–121, 103–111. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hayakawa, K.; Kitaoka, S.; Matsubara, H.; Takayama, H.; Kagiya, Y.; Sugita, Y. The Effect of Preoxidation Atmosphere on Oxidation Behavior and Thermal Cycle Life of Thermal Barrier Coatings. Mater. Sci. Eng. A Struct. 2006, 441, 119–125. [Google Scholar] [CrossRef]
















| Parameter | Unit | APS | HVOF |
|---|---|---|---|
| Arc Current | Amps | 575 | - |
| Primary plasma gas, Nitrogen | NLPM | 35 | - |
| Primary plasma gas, Oxygen | NLPM | - | 800 |
| Secondary plasma gas, Hydrogen | NLPM | 10 | - |
| Carrier gas, Argon | NLPM | 3.0 | 9.0 |
| Powder feed rate | g/min | 55 | - |
| Spraying distance | mm | 90 | - |
| Stirrer | % of max speed | 80 | - |
| Fuel (Kerosene) | Litre/hour | - | 18 |
| Disc rotation | % of max speed | - | 8.0 |
| Temperature (°C) | Hours /Cycles | APS | HVOF |
|---|---|---|---|
| As-applied | - | A | H |
| 1400 (Isothermal test) | 100 h | A100h | H100h |
| 1400 (Thermal cyclic test) | 5 Cycles | A5c | H5c |
| 10 Cycles | A10c | H10c | |
| 1080 Cycles (Until failed) | - | H1080c |
| Tests | Al2O3 Thickness Ratio (%) | Mixed Oxide Ratio (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | 1 | 2 | 3 | Average | 1 | 2 | 3 | Average | |
| Isothermal | APS | 19.40 | 21.89 | 20.90 | 20.73 | 1.32 | 0.32 | 0.43 | 0.69 |
| APS-100 | 29.17 | 33.21 | 31.20 | 31.19 | 6.34 | 7.67 | 6.93 | 6.98 | |
| Thermal cycle | APS-5 | 43.22 | 44.51 | 42.56 | 43.43 | 21.34 | 19.23 | 23.45 | 21.34 |
| APS-10 (failed) | 58.38 | 61.54 | 63.45 | 61.12 | 25.34 | 26.72 | 24.36 | 25.47 | |
| Isothermal | HVOF | 10.65 | 12.34 | 11.54 | 11.51 | 1.18 | 1.83 | 1.96 | 1.66 |
| HVOF-100 | 24.32 | 26.74 | 25.62 | 25.56 | 4.43 | 4.56 | 4.55 | 4.51 | |
| Thermal cycle | HVOF-5 | 29.45 | 30.71 | 35.81 | 31.99 | 7.82 | 8.26 | 9.34 | 8.47 |
| HVOF-10 | 38.94 | 39.68 | 38.77 | 39.13 | 10.34 | 11.28 | 11.54 | 11.05 | |
| HVOF-1080 (failed) | 51.12 | 50.36 | 49.72 | 50.40 | 14.65 | 14.82 | 14.10 | 14.52 | |
| Sample | Topcoat | TGO | Bondcoat |
|---|---|---|---|
| A | 0.2988—t-ZrO2 (111) | - | 0.361—Cr2O3 (012) 0.43 nm—δ Al2O3|(013) |
| H | 0.2902—t-ZrO2 (112) | - | 0.427 nm—δ Al2O3|(013) |
| A100h | 0.2498—t-ZrO2 (110) | 0.258—ZrO2 (002)|0.348—α Al2O3|(012) 0.2096—α Al2O3|(113) 0.484—Spinel (110) 0.5—spinel 0.2095—Ni|(111) | - |
| H100h | 0.258—t-ZrO2 (002) | 0.438—δ Al2O3 (013)|0.451—θ Al2O3|(102) 0.245—δ Al2O3|(311) 0.234—α Al2O3(110) | - |
| A10c | 0.266—c-ZrO2 (111) | 0.5—Spinel 0.2—α Al2O3|(113) 0.4842—Spinel|0.2452—NiO (111) | - |
| H1080c | 0.3148—m-ZrO2|(111) | 0.2905—c-ZrO2|(111)|0.2338—α Al2O3|(110) 0.4919—spinel (110) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahalingam, S.; Manap, A.; Yunus, S.M.; Afandi, N. Thermal Stability of Rare Earth-PYSZ Thermal Barrier Coating with High-Resolution Transmission Electron Microscopy. Coatings 2020, 10, 1206. https://doi.org/10.3390/coatings10121206
Mahalingam S, Manap A, Yunus SM, Afandi N. Thermal Stability of Rare Earth-PYSZ Thermal Barrier Coating with High-Resolution Transmission Electron Microscopy. Coatings. 2020; 10(12):1206. https://doi.org/10.3390/coatings10121206
Chicago/Turabian StyleMahalingam, Savisha, Abreeza Manap, Salmi Mohd Yunus, and Nurfanizan Afandi. 2020. "Thermal Stability of Rare Earth-PYSZ Thermal Barrier Coating with High-Resolution Transmission Electron Microscopy" Coatings 10, no. 12: 1206. https://doi.org/10.3390/coatings10121206