A Facile Method for Preparing a Superhydrophobic Block with Rapid Reparability
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
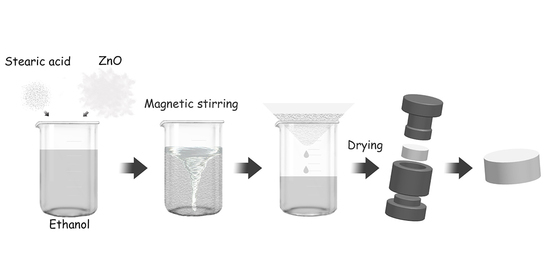
2.2. Method
2.3. Sample Characterization
3. Result and Discussion
3.1. Wettability
3.2. Surface Morphology
3.3. Chemical Composition
3.4. Self-Cleaning Effect
3.5. Durability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, Z.; Liu, W.; Su, B. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar]
- Ueda, E.; Levkin, P. Emerging applications of superhydrophilic-superhydrophobic micropatterns. Adv. Mater. 2013, 25, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Aljallis, E.; Sarshar, M.A.; Datla, R.; Sikka, V.; Jones, A.; Choi, C.-H. Experimental study of skin friction drag reduction on superhydrophobic flat plates in high Reynolds number boundary layer flow. Phys. Fluids 2013, 25, 025103. [Google Scholar] [CrossRef]
- Dong, H.; Cheng, M.; Zhang, Y.; Wei, H.; Shi, F. Extraordinary drag-reducing effect of a superhydrophobic coating on a macroscopic model ship at high speed. J. Mater. Chem. A 2013, 1, 5886–5891. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, H.; Jia, Y.; Liu, J.; Zhang, H.; Wang, R.; Zhang, B.; Zhang, H.; Zhang, Q. Design and preparation of biomimetic polydimethylsiloxane (PDMS) films with superhydrophobic, self-healing and drag reduction properties via replication of shark skin and SI-ATRP. Chem. Eng. J. 2019, 356, 318–328. [Google Scholar] [CrossRef]
- Qian, H.; Xu, D.; Du, C.; Zhang, D.; Li, X.; Huang, L.; Deng, L.; Tu, Y.; Mol, J.; Terryn, H.A. Dual-action smart coatings with a self-healing superhydrophobic surface and anti-corrosion properties. J. Mater. Chem. A 2017, 5, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Ben, S.; Zhou, T.; Ma, H.; Yao, J.; Ning, Y.; Tian, D.; Liu, K.; Jiang, L. Multifunctional magnetocontrollable superwettable microcilia surface for directional droplet manipulation. Adv. Sci. 2019, 6, 1900834. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Chen, L.; Zhang, S.; Huang, H.-X. Superhydrophobic SiC/CNTs coatings with photothermal deicing and passive anti-icing properties. ACS Appl. Mater. Interfaces 2018, 10, 36505–36511. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, H.; Wang, G.; Liu, A. Recent progress in preparation and anti-icing applications of superhydrophobic coatings. Coatings 2018, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Xing, S.; Yuan, Z.; Xiao, J.; Wang, C.; Zeng, J. Preparation and anti-icing of superhydrophobic PVDF coating on a wind turbine blade. Appl. Surf. Sci. 2012, 259, 764–768. [Google Scholar] [CrossRef]
- Renard, C.; Leclercq, L.; Stocco, A.; Cottet, H. Superhydrophobic capillary coatings: Elaboration, characterization and application to electrophoretic separations. J. Chromatogr. A 2019, 1603, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhu, Z.; You, P.; Lin, J.; Cheung, C.F.; Lu, V.L.; Yan, F.; Chan, C.-Y.; Li, G. Plasmonic and superhydrophobic self-decontaminating N95 respirators. ACS Nano 2020, 14, 8846–8854. [Google Scholar] [CrossRef]
- Gupta, N.; Sasikala, S.; Barshilia, H.C. Corrosion study of superhydrophobic magnesium alloy AZ31 surfaces prepared by wet chemical etching process. Nanosci. Nanotechnol. Lett. 2012, 4, 757–765. [Google Scholar] [CrossRef]
- Kumar, A.; Gogoi, B. Development of durable self-cleaning superhydrophobic coatings for aluminium surfaces via chemical etching method. Tribol. Int. 2018, 122, 114–118. [Google Scholar] [CrossRef]
- Zhu, J. A novel fabrication of superhydrophobic surfaces on aluminum substrate. Appl. Surf. Sci. 2018, 447, 363–367. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, X. A new route for fabrication of the corrosion-resistant superhydrophobic surface by milling process. J. Coat. Technol. Res. 2019, 16, 249–255. [Google Scholar] [CrossRef]
- Crick, C.R.; Bear, J.C.; Kafizas, A.; Parkin, I.P. Superhydrophobic photocatalytic surfaces through direct incorporation of titania nanoparticles into a polymer matrix by aerosol assisted chemical vapor deposition. Adv. Mater. 2012, 24, 3505–3508. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, Z.; Chen, L.; Jiang, Y.; Xu, C.; Wu, Z.; Wang, Y.; Peng, C. Porous superhydrophobic and superoleophilic surfaces prepared by template assisted chemical vapor deposition. Surf. Coat. Technol. 2017, 315, 385–390. [Google Scholar] [CrossRef]
- Long, J.; Fan, P.; Gong, D.; Jiang, D.; Zhang, H.; Li, L.; Zhong, M. Superhydrophobic surfaces fabricated by femtosecond laser with tunable water adhesion: From lotus leaf to rose petal. ACS Appl. Mater. Interfaces 2015, 7, 9858–9865. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, C.; Dong, X.; Yin, K.; Zhang, F.; Xie, Z.; Chu, D.; Duan, J. Controllable superhydrophobic aluminum surfaces with tunable adhesion fabricated by femtosecond laser. Opt. Laser Technol. 2018, 102, 25–31. [Google Scholar] [CrossRef]
- Ogihara, H.; Katayama, T.; Saji, T. One-step electrophoretic deposition for the preparation of superhydrophobic silica particle/trimethylsiloxysilicate composite coatings. J. Colloid Interface Sci. 2011, 362, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, D.; Wei, Z.; Chen, J.; Jing, J. Fabrication of superhydrophobic nano-aluminum films on stainless steel meshes by electrophoretic deposition for oil-water separation. Appl. Surf. Sci. 2018, 427, 253–261. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.; Kumar, A.M.; Sadasivuni, K.K.; Xing, R.; Liua, S. Self-cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Qin, X.; Wang, Y.; Liu, C.; Shao, Q.; Wang, N.; Zhang, J.; Wang, Z.; Shen, C.; et al. Superhydrophobic/superoleophilic polycarbonate/carbon nanotubes porous monolith for selective oil adsorption from water. ACS Sustain. Chem. Eng. 2018, 6, 13747–13755. [Google Scholar] [CrossRef]
- Marinaro, G.; Accardo, A.; De Angelis, F.; Dane, T.; Weinhausen, B.; Burghammer, M.; Riekel, C. A superhydrophobic chip based on SU-8 photoresist pillars suspended on a silicon nitride membrane. Lab Chip 2014, 14, 3705–3709. [Google Scholar] [CrossRef] [Green Version]
- Si, W.; Yu, J.; Huang, M.; Ding, C.; Gao, H. Controllable synthesis and photocatalytic activities of cube and hexagonal prism ZnO. Micro Nano Lett. 2012, 7, 1324–1327. [Google Scholar] [CrossRef]
- Ding, N.; Sun, Y.; Chen, B.; Wang, D.; Tao, S.; Zhao, B.; Li, Y. Facile preparation of raspberry-like PS/ZnO composite particles and their antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124867. [Google Scholar] [CrossRef]
- Shi, R.; Yang, P.; Dong, X.; Ma, Q.; Zhang, A. Growth of flower-like ZnO on ZnO nanorod arrays created on zinc substrate through low-temperature hydrothermal synthesis. Appl. Surf. Sci. 2013, 264, 162–170. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, L.; Wu, D. Fabrication of superhydrophobic surfaces from microstructured ZnO-based surfaces via a wet-chemical route. Langmuir 2005, 21, 2665–2667. [Google Scholar] [CrossRef]
- Qing, Y.-Q.; Yang, C.-N.; Sun, Y.-Z.; Zheng, Y.-S.; Shang, Y.; Liu, C. Simple method for preparing ZnO superhydrophobic surfaces with micro/nano roughness. J. Adhes. Sci. Technol. 2015, 29, 2153–2159. [Google Scholar] [CrossRef]
- Sutha, S.; Kumar, R.T.R.; Raj, B.; Ravi, K.R. Ultrasonic-assisted fabrication of superhydrophobic ZnO nanowall films. Bull. Mater. Sci. 2017, 40, 505–511. [Google Scholar] [CrossRef]
- Wei, X.-L.; Li, N.; An, J.-F.; Huo, C.-F.; Liu, H.; Yang, R.; Li, X.; Chao, Z.-S. Synthesis of superhydrophobic flower-like ZnO on nickel foam. CrystEngComm 2020, 22, 205–212. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Zhang, Y. Fabrication and characterization of superhydrophobicity ZnO nanoparticles with two morphologies by using stearic acid. Mater. Res. Express 2019, 6, 1150d1. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef]
- Masood, M.T.; Zahid, M.; Goldoni, L.; Ceseracciu, L.; Athanassiou, A.; Bayer, I.S. Highly transparent polyethylcyanoacrylates from approved eco-friendly fragrance materials demonstrating excellent fog-harvesting and anti-wear properties. ACS Appl. Mater. Interfaces 2018, 10, 34573–34584. [Google Scholar] [CrossRef]
- Bayer, I.S. On the durability and wear resistance of transparent superhydrophobic coatings. Coatings 2017, 7, 12. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Liao, K. A Facile Method for Preparing a Superhydrophobic Block with Rapid Reparability. Coatings 2020, 10, 1202. https://doi.org/10.3390/coatings10121202
Zhu J, Liao K. A Facile Method for Preparing a Superhydrophobic Block with Rapid Reparability. Coatings. 2020; 10(12):1202. https://doi.org/10.3390/coatings10121202
Chicago/Turabian StyleZhu, Jiyuan, and Kaijin Liao. 2020. "A Facile Method for Preparing a Superhydrophobic Block with Rapid Reparability" Coatings 10, no. 12: 1202. https://doi.org/10.3390/coatings10121202