A Promising Nano-Insulating-Oil for Industrial Application: Electrical Properties and Modification Mechanism
Abstract
:1. Introduction
1.1. Long-Term Stability
1.2. Decrease in Negative Impulse Breakdown Performance
1.3. Significant Increase in Dielectric Loss
2. Experimental Setup
2.1. Preparation of Nanofluids
2.2. AC Breakdown Tests
2.3. Lightning Impulse Breakdown Tests
2.4. Frequency Domain Dielectric Spectrum Tests
2.5. TSC Tests
2.6. Transmission Electron Microscopy (TEM)
2.7. Particle Size Distribution and Zeta Potential Tests
3. Results
3.1. Zeta Potential and Particle Size Distribution of CMIO
3.2. Micromorphology of Nano-C60
3.3. Breakdown Characteristics of FO and CMIO
3.4. Dielectric Characteristics of FO and CMIO
4. Analysis and Discussion
4.1. Trap Characteristics
4.2. Adsorption of Charge Carriers and Percolation Threshold Phenomenon
4.3. Relaxation Time Constant and Electrical Potential Distribution of C60 Nanoparticles
- In this model, the applied electric field E0 is uniform. In the TSC test, the small shim in the oil tank (Figure 3) affects the uniformity of the electric field distribution.
- The nanoparticles (or nanoparticle agglomerates) are assumed to be standard spheres in this model, and thus their morphology may be different from that of C60 nanoparticles present in CMIO.
- More importantly, we believe that the adsorption of carriers by C60 in CMIO is not only the effect of the electric potential wells produced by the induced dipole consisting of C60 nanoparticles under a high electric field, but also the electronegativity effect of C60. We will further discuss the effect of C60 electronegativity on electron adsorption in Section 4.4.
4.4. Electronegativity of C60
4.5. Absorption of Photons by C60
4.6. Summary of the Mechanism of C60
- The addition of C60 nanoparticles deepens the trap energy level and increases the trap density in insulating oil. In CMIO, the frequency of electrons entering the trap is higher than the frequency of electrons leaving the trap, and the capture ability of the electron trap is strong. Carrier mobility in CMIO is lower than that in FO. The number of electrons trapped by the deep and dense traps increases.
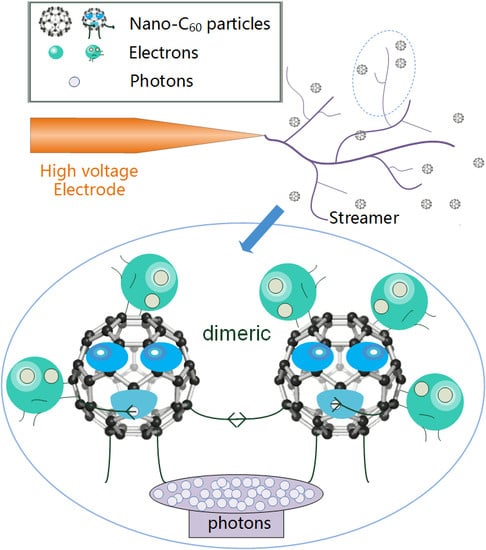
- C60 nanoparticles have a strong ability to absorb electrons. As C60 captures electrons, it reduces the number of electrons that can participate in the formation of electron avalanches under an electric field, thus weakening the development of streamers and increasing the breakdown voltage of CMIO.
- C60 molecules can absorb photons generated in the process of streamer development though photon absorption polymerization (most are dimers), which weakens the process of photoionization, and thus disrupts the development of streamers and improves the breakdown performance of CMIO. We believe that the absorption of photons by C60 is the reason for the improvement in the negative impulse breakdown performance of CMIO.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, S.U.S.; Eastman, J. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, San Francisco, CA, USA, 12–17 November 1995; pp. 66–74. [Google Scholar]
- Fedele, L.; Colla, L.; Bobbo, S.; Barison, S.; Agresti, F. Experimental stability analysis of different water-based nanofluids. Nanoscale. Res. Lett. 2011, 6, 300–308. [Google Scholar] [CrossRef]
- Viali, W.R.; Alcantara, G.B.; Sartoratto, P.P.C.; Soler, M.A.G. Investigation of the molecular surface coating on the stability of Insulating magnetic oils. J. Phys. Chem. C 2010, 114, 179–188. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, H.S.; Oh, J.M. Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr. Appl. Phys. 2008, 8, 710–712. [Google Scholar]
- Segal, V.; Hjortsberg, A.; Rabinovich, A.; Nattrass, D.; Raj, K. AC (60 Hz) and impulse breakdown strength of a colloidal fluid based on transformer oil and magnetite nanoparticles. In Proceedings of the IEEE International Symposium on Electrical Insulation, Arlington, VA, USA, 7–10 June 1998; pp. 619–622. [Google Scholar]
- Sima, W.; Cao, X.; Yang, Q. Preparation of three transformer oil-based nanofluids and comparison of their impulse breakdown characteristics. Nanosci. Nanotech. Let. 2014, 6, 250–256. [Google Scholar] [CrossRef]
- Segal, V.; Nattrass, D.; Raj, K. Accelerated thermal aging of petroleum-based ferrofluids. J. Magn. Mate. 1999, 201, 70–72. [Google Scholar] [CrossRef]
- Wang, Q.; Rafiq, M.; Lv, Y.; Li, C.; Yi, K.J. Preparation of three types of transformer oil-based nanofluids and comparative study on the effect of nanoparticle concentrations on insulating property of transformer oil. J. Nanotechnol. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Rafiq, M.; Li, C.; Khan, I.; Hu, Z.; Lv, Y.; Yi, K. Preparation and breakdown properties of mineral oil based alumina nanofluids. In Proceedings of the IEEE International Conference on Emerging Technologies, Peshawar, Pakistan, 19–20 December 2015; pp. 1–3. [Google Scholar]
- Lv, Y.; Ge, Y.; Li, C. Effect of TiO2 nanoparticles on streamer propagation in transformer oil under lightning impulse voltage. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2110–2115. [Google Scholar] [CrossRef]
- Rafiq, M.; Li, C.; Lv, Y.; Yi, K.; Ikram, A. Breakdown characteristics of transformer oil based silica nanofluids. In Proceedings of the 19th International Multi-Topic Conference, Islamabad, Pakistan, 5–6 December 2016; pp. 302–305. [Google Scholar]
- Liu, R.; Pettersson, L.A.A.; Auletta, T.; Hjortstam, O. Fundamental research on the application of nano dielectrics to transformers. In Proceedings of the Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 16–19 October 2011; pp. 423–427. [Google Scholar]
- Rakov, V.A. Lightning parameters for engineering applications (keynote speech). In Proceedings of the 2010 Asia-Pacific International Symposium on Electromagnetic Compatibility, Beijing, China, 12–16 April 2010; pp. 1120–1123. [Google Scholar]
- Sun, P.; Sima, W.; Zhang, D.; Jiang, X.; Chen, Q.; Chen, J. Impact of wavefront time of microsecond impulse on the breakdown voltage of oil impregnated paper: Mechanism analysis. IEEE Trans. on Dielectr. Electr. Insul. 2018, 25, 1706–1715. [Google Scholar] [CrossRef]
- Sartoratto, P.P.C.; Neto, A.V.S.; Lima, E.C.D. Preparation and electrical properties of oil-based magnetic fluids. J. Appl. Phys. 2005, 97, 10Q917. [Google Scholar] [CrossRef]
- Mergos, J.; Athanassopoulou, M.; Argyropoulos, T. Dielectric properties of nanopowder dispersions in paraffin oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1502–1507. [Google Scholar] [CrossRef]
- Wang, X.; Woo, H.K.; Wang, L. Vibrational cooling in a cold ion trap: Vibrationally resolved photoelectron spectroscopy of cold C60(-) anions. J. Chem. Phys. 2005, 123, 051106. [Google Scholar] [CrossRef]
- Sun, P.; Sima, W.; Chen, J. An application area of C60: Overall improvement of insulating oil’s electrical performance. Appl. Phys. Lett. 2018, 112, 142902. [Google Scholar] [CrossRef]
- Yanilkin, V.V.; Gubskaya, V.P.; Morozov, V.I. Electrochemistry of fullerenes and their derivatives. Russ. J. Electrochem. 2003, 39, 1147–1165. [Google Scholar] [CrossRef]
- Rao, A.; Zhou, P.; Wang, K. Photoinduced polymerization of solid C60 films. Science 1993, 259, 955–957. [Google Scholar] [CrossRef]
- Sivaraman, N.; Dhamodaran, R.; Kaliappan, I. Solubility of C60 in organic solvents. J. Org. Chem. 1992, 57, 6077–6079. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Tse, D.S.; Malhotra, R. Solubility of fullerene (C60) in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Dielectric Breakdown Voltage of Insulating Oils of Petroleum Origin under Impulse Conditions; ASTM D3487-2000(II); ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- Iio, Y.; Kurihara, K.; Matsuyama, F. Photopolymerization of C60 crystal synthesized from organic solution. J. Phys. 2009, 159, 012019. [Google Scholar]
- Akhlaghi, S.; Pourrahimi, A.M. Effects of ageing conditions on degradation of acrylonitrile butadiene rubber filled with heat-treated ZnO star-shaped particles in rapeseed biodiesel. Polym. Degrad. Stabil. 2017, 138, 27–39. [Google Scholar] [CrossRef] [Green Version]
- IEC. Insulating Liquids–Determination of the Breakdown Voltage at Power Frequency–Test Method; IEC 60156-1995; IEC: Geneva, Switzerland, 1995. [Google Scholar]
- Liu, Q.; Wang, Z. Breakdown and withstand strengths of ester transformer liquids in a quasi-uniform field under impulse voltages. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 571–579. [Google Scholar] [CrossRef]
- Chen, R. Analysis of Thermally Stimulated Processes; Pergamon: Oxford, NY, USA, 1981. [Google Scholar]
- Zhong, Y.; Lv, Y.; Li, C. Insulating properties and charge characteristics of natural ester fluid modified by TiO2 semiconductive nanoparticles. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 135–140. [Google Scholar] [CrossRef]
- Vandsburger, L. Synthesis and Covalent Surface Modification of Carbon Nanotubes for Preparation of Stabilized Nanofluid Suspensions. Ph.D. Thesis, McGill University, , Montreal, QC, Canada, 2009. [Google Scholar]
- Ghadimi, A.; Saidur, R.; Metselaar, H.S.C. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Mass. Tran. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Chen, K.L.; Elimelech, M. Relating Colloidal Stability of Fullerene (C60) Nanoparticles to Nanoparticle Charge and Electrokinetic Properties. Environ. Sci. Technol. 2009, 43, 7270–7276. [Google Scholar] [CrossRef] [PubMed]
- Pourrahimi, A.M.; Liu, D.; Pallon, L.K.H. Water-based synthesis and cleaning methods for high purity ZnO nanoparticles – comparing acetate, chloride, sulphate and nitrate zinc salt precursors. RSC. Adv. 2014, 4, 35568. [Google Scholar] [CrossRef]
- Gallagher, S.H.; Armstrong, R.S.; Lay, P.A. Solvent Effects on the Electronic Spectrum of C60. J. Phys. Chem. 1995, 99, 5817–5825. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, K.S.; Jang, S.P. Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int. J. Heat. Mass. Tran. 2008, 51, 2651–2656. [Google Scholar] [CrossRef]
- Sima, W.; Sun, P.; Yang, Q. Study on the accumulative effect of repeated lightning impulses on insulation characteristics of transformer oil impregnated paper. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1933–1941. [Google Scholar] [CrossRef]
- Sami, S.; Haase, P.A.B.; Alessandri, R. Can the dielectric constant of fullerene derivatives be enhanced by side chain manipulation? a predictive first principles computational study. J. Phys. Chem. A. 2018, 122, 3919–3926. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.D.; Mazumdar, P.S.; Gartia, R.K. The determination of the trapping parameters of a thermoluminescence peak by using the Kirsh method. J. Phys. D: Appl. Phys. 1998, 31, 231–234. [Google Scholar] [CrossRef]
- Hoogenboom, J.E.; Devries, W.; Dielhof, J.B.J. Computerized analysis of glow curves from thermally activated processes. Appl. Phys. 1988, 64, 3193–3200. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, X.; Fan, Y. A new method of auto-separating thermally stimulated current. J. App. Phys. 1992, 72, 4254–4257. [Google Scholar] [CrossRef]
- Sun, P.; Sima, W.; Zhang, D.; Jiang, X.; Zhang, H.; Yin, Z. Failure characteristics and mechanism of nano-modified oil-Impregnated paper subjected to repeated impulse voltage. Nanomaterials 2018, 8, 504. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Olsson, R.T.; Hedenqvist, M.S. The Role of Interfaces in Polyethylene/Metal-Oxide Nanocomposites for Ultrahigh-Voltage Insulating. Materials. Advanced. Mate. 2018, 30, 1703624. [Google Scholar] [CrossRef]
- Min, D.; Cho, M.G.; Khan, A.R. Charge transport properties of dielectrics revealed by isothermal surface potential decay. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1465–1473. [Google Scholar] [CrossRef]
- Sessler, G.M.; Figueiredo, M.T.; Ferreira, G.F.L. Models of charge transport in electron-beam irradiated insulators. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 192–202. [Google Scholar] [CrossRef]
- Kao, K.C. Dielectric Phenomena in Solids; Elsevier Academic: San Diego, CA, USA, 2004; pp. 381–509. [Google Scholar]
- Min, D.; Li, S. IEEE Trans. Simulation on the influence of bipolar charge injection and trapping on surface potential decay of polyethylene. Dielectr. Electr. Insul. 2014, 21, 1627–1636. [Google Scholar] [CrossRef]
- Nelson, J.K. Dielectric Polymer Nanocomposites; Springer: New York, NY, USA, 2010. [Google Scholar]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; Taylor: London, UK, 1992. [Google Scholar]
- Balberg, I.; Azulay, D.; Toker, D. Percolation and tunneling in composite materials. Int. J. Mod. Phys. B 2012, 18, 2091–2121. [Google Scholar] [CrossRef]
- Hoang, A.T.; Pallon, L.; Liu, D.; Serdyuk, Y.V.; Gubanski, S.M.; Gedde, U.W. Charge Transport in LDPE Nanocomposites Part I—Experimental Approach. Polymers 2016, 8, 87. [Google Scholar] [CrossRef]
- Hong, J.I.; Schadler, L.S.; Siegel, R.W. Rescaled electrical properties of ZnO/low density polyethylene nanocomposites. Appl. Phys. Lett. 2003, 82, 1956. [Google Scholar] [CrossRef]
- Peimo, H.; Yabo, X.; Xuejia, Z. Electrical conductivity studies of a pure C60 single crystal. J. Phys. Condens. Mat. 1993, 5, 7013–7016. [Google Scholar] [CrossRef]
- Hwang, J.G.; Zahn, M.; Sullivan, O.; Francis, M. Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J. Appl. Phys. 2010, 107, 014310. [Google Scholar] [CrossRef] [Green Version]
- Takada, T.; Hayase, Y.; Tanaka, Y. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 152–160. [Google Scholar] [CrossRef]



















| NP 1/Oil System | Nanoparticle Type | Concentration | Size of NP | Percentage Increase | Ref |
|---|---|---|---|---|---|
| Fe3O4/U-60 2 | Conductor | NM 6 | <10 nm | −9.41% | [5] |
| Fe3O4/Nytro 3 | Conductor | NM | <10 nm | −2.26% | [5] |
| Fe3O4/#25MO 4 | Conductor | 0.03 g/L | 20 nm | −15.82% | [6] |
| TiO2/#25MO | Semiconductor | 0.01 g/L | 20 nm | −3.39% | [6] |
| Al2O3/#25MO | Insulator | 0.02 g/L | 20 nm | −2.53% | [6] |
| (Fe3O4/ MO) Aged 5 | Conductor | NM | <10 nm | −7.75% | [7] |
| TiO2/#25MO | Semiconductor | 5% w/v | <50 nm | −4.49% | [8] |
| Al2O3/#25MO | Insulator | 5% w/v | <50 nm | −7.34% | [8] |
| Fe3O4/#25MO | Conductor | 5% w/v | <50 nm | −6.12% | [8] |
| Al2O3/#25MO | Insulator | 20% w/v | NM | −9.98% | [9] |
| TiO2/#25MO | Semiconductor | 0.075% v/v | <10 nm | −6.84% | [10] |
| SiO2/#25MO | Insulator | 20% w/v | NM | −5.93% | [11] |
| ZnO/#25MO | Semiconductor | 0.0475% v/v | 5~20 nm | −34.4% | [12] |
| Al2O3/#25MO | Insulator | 0.1425% v/v | 5~20 nm | −13.0% | [12] |
| Volume Fraction (%) | Loss Factor | Growth Multiple of Loss Factor | Dielectric Strength (kV) |
|---|---|---|---|
| 0.0 | 0.00366 | 1 | 70 |
| 0.800 | 2.389 | 653 | - |
| 0.016 | 0.399 | 109 | 79 |
| 0.0080 | 0.1868 | 51 | 72 |
| 0.0040 | 0.1274 | 35 | 70 |
| Measurement times | 1 | 2 | 3 | 4 | 5 | 6 | Average value/ mV |
| Zeta potential/mV | −43.0 | −40.6 | −41.5 | −39.7 | −38.3 | −45.0 | −41.3 |
| Measurement times | 7 | 8 | 9 | 10 | 11 | 12 | Standard deviation |
| Zeta potential/mV | −45.5 | −41.1 | −41.9 | −39.7 | −38.9 | −41.0 | 2.241 |
| Trap Parameter | FO | CMIO |
|---|---|---|
| Peak Current (I)/pA | 2.68 | 4.84 |
| Peak Current (II)/pA | 1.25 | 1.25 |
| Trap level (I)/eV | 0.505 | 0.718 |
| Trap level (II)/eV | 0.426 | 0.426 |
| Q (I)/nC | 3.63 | 5.12 |
| Q (II)/nC | 1.30 | 1.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Sun, P.; Sima, W.; Shao, Q.; Ye, L.; Li, C. A Promising Nano-Insulating-Oil for Industrial Application: Electrical Properties and Modification Mechanism. Nanomaterials 2019, 9, 788. https://doi.org/10.3390/nano9050788
Chen J, Sun P, Sima W, Shao Q, Ye L, Li C. A Promising Nano-Insulating-Oil for Industrial Application: Electrical Properties and Modification Mechanism. Nanomaterials. 2019; 9(5):788. https://doi.org/10.3390/nano9050788
Chicago/Turabian StyleChen, Jiaqi, Potao Sun, Wenxia Sima, Qianqiu Shao, Lian Ye, and Chuang Li. 2019. "A Promising Nano-Insulating-Oil for Industrial Application: Electrical Properties and Modification Mechanism" Nanomaterials 9, no. 5: 788. https://doi.org/10.3390/nano9050788