Boron Nitride Doped Polyhydroxyalkanoate/Chitosan Nanocomposite for Antibacterial and Biological Applications
Abstract
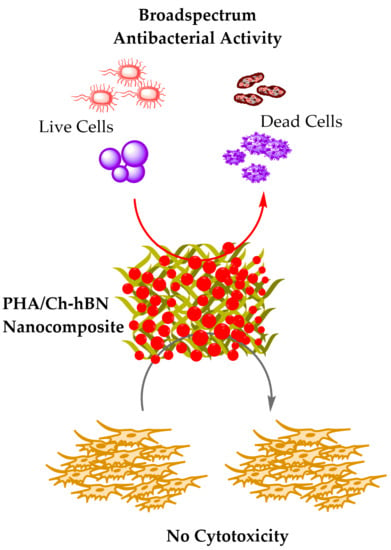
:1. Introduction
2. Materials and Methods
2.1. Preparation of Precursor Solution
2.2. Solvent Casting
2.3. Antibacterial Assays
2.4. Cytotoxicity Assays
3. Results
3.1. Morphological Analysis
3.2. FT-IR
3.3. Thermal Gravimetric Analysis (TGA)
4. Antibacterial Analysis
5. Cell Cytotoxicity Assay against HaCaT Cell Lines
6. Comparison of Antibacterial Efficiencies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in europe: An overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Pathogenesis of staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Paterson, I.K.; Hoyle, A.; Ochoa, G.; Baker-Austin, C.; Taylor, N.G. Optimising antibiotic usage to treat bacterial infections. Sci. Rep. 2016, 6, 37853. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Lakshminarayanan, R.; Ye, E.; Young, D.J.; Li, Z.; Loh, X.J. Recent advances in the development of antimicrobial nanoparticles for combating resistant pathogens. Adv. Healthc. Mater. 2018, 1701400. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against gram-positive and gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef]
- Sivakumar, P.; Lee, M.; Kim, Y.-S.; Shim, M.S. Photo-triggered antibacterial and anticancer activities of zinc oxide nanoparticles. J. Mater. Chem. B 2018, 6, 4852–4871. [Google Scholar] [CrossRef]
- Merlo, A.; Mokkapati, V.R.; Pandit, S.; Mijakovic, I. Boron nitride nanomaterials: Biocompatibility and bio-applications. Biomater. Sci. 2018, 6, 2298–2311. [Google Scholar] [CrossRef]
- Song, L.; Ci, L.; Lu, H.; Sorokin, P.B.; Jin, C.; Ni, J.; Kvashnin, A.G.; Kvashnin, D.G.; Lou, J.; Yakobson, B.I. Large scale growth and characterization of atomic hexagonal boron nitride layers. Nano Lett. 2010, 10, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Ricotti, L.; Moscato, S.; Bertoni, G.; Falqui, A.; Berrettini, S.; Petrini, M.; Mattoli, V. Enhancement of neurite outgrowth in neuronal-like cells following boron nitride nanotube-mediated stimulation. ACS Nano 2010, 4, 6267–6277. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Rouzaud, F.; Richard, T.; Keshri, A.K.; Bakshi, S.R.; Kos, L.; Agarwal, A. Boron nitride nanotube reinforced polylactide–polycaprolactone copolymer composite: Mechanical properties and cytocompatibility with osteoblasts and macrophages in vitro. Acta Biomater. 2010, 6, 3524–3533. [Google Scholar] [CrossRef]
- Lahiri, D.; Singh, V.; Benaduce, A.P.; Seal, S.; Kos, L.; Agarwal, A. Boron nitride nanotube reinforced hydroxyapatite composite: Mechanical and tribological performance and in-vitro biocompatibility to osteoblasts. J. Mech. Behav. Biomed. Mater. 2011, 4, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Kıvanç, M.; Barutca, B.; Koparal, A.T.; Göncü, Y.; Bostancı, S.H.; Ay, N. Effects of hexagonal boron nitride nanoparticles on antimicrobial and antibiofilm activities, cell viability. Mater. Sci. Eng. C 2018, 91, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Mohammed, I.A.; Shahabuddin, S.; Khanam, R.; Saidur, R. Synthesis, characterization and antibacterial activity of novel poly (silyl ether) s based on palm and soy oils. Polímeros 2018. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.Y.; Sudesh, K. Polyhydroxyalkanoates: Bio-based microbial plastics and their properties. Malaysian Polym. J. 2007, 2, 31–57. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. Recent advances and challenges towards sustainable polyhydroxyalkanoate (pha) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Vijayendra, S.; Shamala, T. Film forming microbial biopolymers for commercial applications—A review. Crit. Rev. Biotechnol. 2014, 34, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Gumel, A.; Annuar, M. Nanocomposites of polyhydroxyalkanoates (phas). In Polyhydroxyalkanoate (PHA) based Blends, Composites and Nanocomposites; The Royal Society of Chemistry: London, UK, 2014; pp. 98–118. [Google Scholar]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Loh, X.J. Polyhydroxyalkanoates: Opening doors for a sustainable future. NPG Asia Mater. 2016, 8, e265. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G.-Q. Polyhydroxyalkanoate (pha) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1045. [Google Scholar] [CrossRef]
- Mukheem, A.; Muthoosamy, K.; Manickam, S.; Sudesh, K.; Shahabuddin, S.; Saidur, R.; Akbar, N.; Sridewi, N. Fabrication and characterization of an electrospun pha/graphene silver nanocomposite scaffold for antibacterial applications. Materials 2018, 11, 1673. [Google Scholar] [CrossRef]
- Saikia, C.; Gogoi, P.; Maji, T. Chitosan: A promising biopolymer in drug delivery applications. J. Mol. Genet. Med. S 2015, 4, 006. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Sarih, N.M.; Ismail, F.H.; Shahid, M.M.; Huang, N.M. Synthesis of chitosan grafted-polyaniline/co 3 o 4 nanocube nanocomposites and their photocatalytic activity toward methylene blue dye degradation. RSC Adv. 2015, 5, 83857–83867. [Google Scholar] [CrossRef]
- Karbasi, S.; Khorasani, S.N.; Ebrahimi, S.; Khalili, S.; Fekrat, F.; Sadeghi, D. Preparation and characterization of poly (hydroxy butyrate)/chitosan blend scaffolds for tissue engineering applications. Adv. Biomed. Res. 2016, 5. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagheer, M.; Siddiqui, R.; Iqbal, J.; Khan, N.A. Black cobra (naja naja karachiensis) lysates exhibit broad-spectrum antimicrobial activities. Pathog. Global Health 2014, 108, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Syed, S. Polyaniline based Nanocomposites as Adsorbents and Photocatalysts in the Removal of Organic dyes/Syed Shahabuddin. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Salim, Y.S.; Chan, C.H.; Sudesh, K.; Gan, S.N. Influence of Thermal Treatment on the Molecular Weights of Polyhydroxyalkanoate Containing 3-hydroxyhexanoate. Adv. Mater. Res. 2013, 812, 250–253. [Google Scholar] [CrossRef]
- Shamala, T.; Divyashree, M.; Davis, R.; Kumari, K.L.; Vijayendra, S.; Raj, B. Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by fourier transform infrared spectroscopy and scanning electron microscopy. Indian J. Microbiol. 2009, 49, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Kisku, S.K.; Swain, S.K. Synthesis and characterization of chitosan/boron nitride composites. J. Am. Ceram. Soc. 2012, 95, 2753–2757. [Google Scholar] [CrossRef]
- Salehirad, M.; Nikje, M.M.A. Synthesis and characterization of exfoliated polystyrene grafted hexagonal boron nitride nanosheets and their potential application in heat transfer nanofluids. Iran. Polym. J. 2017, 26, 467–480. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Khanam, R.; Khalid, M.; Sarih, N.M.; Ching, J.J.; Mohamad, S.; Saidur, R. Synthesis of 2d boron nitride doped polyaniline hybrid nanocomposites for photocatalytic degradation of carcinogenic dyes from aqueous solution. Arab. J. Chem. 2018. [Google Scholar] [CrossRef]
- Firestein, K.L.; Leybo, D.V.; Steinman, A.E.; Kovalskii, A.M.; Matveev, A.T.; Manakhov, A.M.; Sukhorukova, I.V.; Slukin, P.V.; Fursova, N.K.; Ignatov, S.G. Bn/ag hybrid nanomaterials with petal-like surfaces as catalysts and antibacterial agents. Beilstein J. Nanotechnol. 2018, 9, 250–261. [Google Scholar] [CrossRef]
- Shoeb, M.; Mobin, M.; Rauf, M.A.; Owais, M.; Naqvi, A.H. In vitro and in vivo antimicrobial evaluation of graphene–polyindole (gr@ pin) nanocomposite against methicillin-resistant staphylococcus aureus pathogen. ACS Omega 2018, 3, 9431–9440. [Google Scholar] [CrossRef]
- Nasr, M.; Soussan, L.; Viter, R.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. High photodegradation and antibacterial activity of bn–ag/tio 2 composite nanofibers under visible light. New J. Chem. 2018, 42, 1250–1259. [Google Scholar] [CrossRef]
- Parra, C.; Montero-Silva, F.; Henríquez, R.; Flores, M.; Garín, C.; Ramírez, C.; Moreno, M.; Correa, J.; Seeger, M.; Häberle, P. Suppressing bacterial interaction with copper surfaces through graphene and hexagonal-boron nitride coatings. ACS Appl. Mater. Interfaces 2015, 7, 6430–6437. [Google Scholar] [CrossRef] [PubMed]
- Nithya, J.S.M.; Pandurangan, A. Aqueous dispersion of polymer coated boron nitride nanotubes and their antibacterial and cytotoxicity studies. RSC Adv. 2014, 4, 32031–32046. [Google Scholar] [CrossRef]









| Test Samples | Antibacterial Activity against E. coli K1 | Antibacterial Activity against MRSA |
|---|---|---|
| PHA/Ch | - | - |
| PHA/Ch-hBN (0.1 wt%) | + | + |
| PHA/Ch-hBN (0.5 wt%) | + | + |
| PHA/Ch-hBN (1 wt%) | + | + |
| Gentamicin | + | + |
| Nano Composites | Conc. | % Reduction | Time (h) | Proposed Applications | Ref. | |
|---|---|---|---|---|---|---|
| E.coli | MRSA | |||||
| BN/Ag | 70 mg/L | 100 | - | 3 | Eternal catalyst and antibacterial | [39] |
| Gr-Pln | 5 mg/mL | - | 92 | - | Antibacterial applications | [40] |
| BNAg/TiO2 | 2 mg/mL | 100 | - | 3 | Photodegradation and antibacterial applications | [41] |
| Cu-Go/hBN | - | 100 | - | 24 | Biology and medical applications | [42] |
| PEI/BNNT | 1 mg/mL | 95 | 90 | 2 | Nano vector for targeted drug delivery system | [43] |
| PHA/GAg | - | 82 | 60 | 2 | Antibacterial and sanitization application | [26] |
| PHA/Ch-hBN | 1 mg/mL | 92 | 97 | 2 | Antibacterial and biological applications | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukheem, A.; Shahabuddin, S.; Akbar, N.; Miskon, A.; Muhamad Sarih, N.; Sudesh, K.; Ahmed Khan, N.; Saidur, R.; Sridewi, N. Boron Nitride Doped Polyhydroxyalkanoate/Chitosan Nanocomposite for Antibacterial and Biological Applications. Nanomaterials 2019, 9, 645. https://doi.org/10.3390/nano9040645
Mukheem A, Shahabuddin S, Akbar N, Miskon A, Muhamad Sarih N, Sudesh K, Ahmed Khan N, Saidur R, Sridewi N. Boron Nitride Doped Polyhydroxyalkanoate/Chitosan Nanocomposite for Antibacterial and Biological Applications. Nanomaterials. 2019; 9(4):645. https://doi.org/10.3390/nano9040645
Chicago/Turabian StyleMukheem, Abdul, Syed Shahabuddin, Noor Akbar, Azizi Miskon, Norazilawati Muhamad Sarih, Kumar Sudesh, Naveed Ahmed Khan, Rahman Saidur, and Nanthini Sridewi. 2019. "Boron Nitride Doped Polyhydroxyalkanoate/Chitosan Nanocomposite for Antibacterial and Biological Applications" Nanomaterials 9, no. 4: 645. https://doi.org/10.3390/nano9040645