Facile Synthesis of Triphenylamine Based Hyperbranched Polymer for Organic Field Effect Transistors
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. General Characterization
2.3. Synthesis
2.3.1. Synthesis of Tris(4-bromophenyl)amine
2.3.2. Synthesis of Polymer Tris[(4-phenyl)amino-alt-4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b;4,5-b’]dithiophene] (PTPABDT)
2.4. Organic Field Effect Transistors (OFET) Fabrication
3. Results and Discussion
3.1. PTPABDT Synthesis and Characterization
3.2. Optical Properties
3.3. Electrochemical Properties
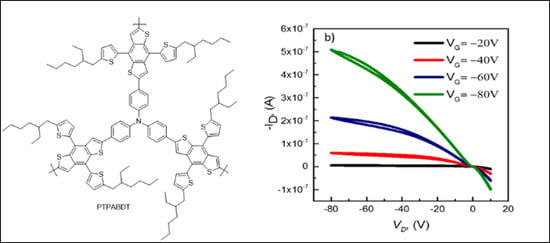
3.4. I-V Characteristics of PTPABDT Based OFETs Device.
3.5. Interfacial Electron Transport Properties: EIS-Nyquist Plot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baeg, K.-J.; Caironi, M.; Noh, Y.-Y. Toward Printed Integrated Circuits based on Unipolar or Ambipolar Polymer Semiconductors. Adv. Mater. 2013, 25, 4210–4244. [Google Scholar] [CrossRef] [PubMed]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirringhaus, H. Device Physics of Solution-Processed Organic Field-Effect Transistors. Adv. Mater. 2005, 17, 2411–2425. [Google Scholar] [CrossRef]
- Opoku, H.; Nketia-Yawson, B.; Shin, E.-S.; Noh, Y.-Y. Organic field-effect transistors processed by an environmentally friendly non-halogenated solvent blend. J. Mater. Chem. C 2018, 6, 661–667. [Google Scholar] [CrossRef]
- Ahmad, S. Organic semiconductors for device applications: Current trends and future prospects. J. Polym. Eng. 2014, 34, 279. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, C.; Khim, D.; Noh, Y.-Y. Development of high-performance printed organic field-effect transistors and integrated circuits. Phys. Chem. Chem. Phys. 2015, 17, 26553–26574. [Google Scholar] [CrossRef]
- McCulloch, I. Rolling out organic electronics. Nat. Mater. 2005, 4, 583–584. [Google Scholar] [CrossRef]
- Yuan, Y.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.B.; Chen, J.; Nordlund, D.; Toney, M.F.; Huang, J.; Bao, Z. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5, 3005. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yao, Y.; Shan, B.; Gu, X.; Liu, D.; Liu, J.; Xu, J.; Zhao, N.; Hu, W.; Miao, Q. Electron Mobility Exceeding 10 cm2 V−1 s−1 and Band-Like Charge Transport in Solution-Processed n-Channel Organic Thin-Film Transistors. Adv. Mater. 2016, 28, 5276–5283. [Google Scholar] [CrossRef]
- Kim, G.; Kang, S.-J.; Dutta, G.K.; Han, Y.-K.; Shin, T.J.; Noh, Y.-Y.; Yang, C. A Thienoisoindigo-Naphthalene Polymer with Ultrahigh Mobility of 14.4 cm2/V·s That Substantially Exceeds Benchmark Values for Amorphous Silicon Semiconductors. J. Am. Chem. Soc. 2014, 136, 9477–9483. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.F.; Treat, N.D.; Zhang, W.; Fei, Z.; Wyatt-Moon, G.; Faber, H.; Vourlias, G.; Patsalas, P.A.; Solomeshch, O.; Tessler, N.; et al. Small Molecule/Polymer Blend Organic Transistors with Hole Mobility Exceeding 13 cm2 V−1 s−1. Adv. Mater. 2016, 28, 7791–7798. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Park, W.-T.; Park, J.-I.; Yun, Y.; Gu, X.; Lee, J.; Noh, Y.-Y. Thiophene-Thiazole-Based Semiconducting Copolymers for High-Performance Polymer Field-Effect Transistors. ACS Appl. Mater. Interfaces 2017, 9, 38728–38736. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Guo, Y.; Hu, W.; Liu, Y. Design and effective synthesis methods for high-performance polymer semiconductors in organic field-effect transistors. Mater. Chem. Front. 2017, 1, 2423–2456. [Google Scholar] [CrossRef]
- Bathula, C.; Opoku, H.; Kadam, A.; Shrestha, N.K.; Lee, T.; Noh, Y.-Y. Facile synthesis and optoelectronic exploration of silylthiophene substituted benzodithiophene polymer for organic field effect transistors. J. Organomet. Chem. 2019, 880, 317–321. [Google Scholar] [CrossRef]
- Song, D.H.; Choi, M.H.; Kim, J.Y.; Jang, J.; Kirchmeyer, S. Process optimization of organic thin-film transistor by ink-jet printing of DH4T on plastic. Appl. Phys. Lett. 2007, 90, 053504. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, Y.D.; Jang, Y.; Yang, H.; Kim, Y.H.; Han, J.I.; Moon, D.G.; Park, S.; Chang, T.; Chang, C.; et al. Enhancement of Field-Effect Mobility Due to Surface-Mediated Molecular Ordering in Regioregular Polythiophene Thin Film Transistors. Adv. Funct. Mater. 2005, 15, 77–82. [Google Scholar] [CrossRef]
- Shin, E.-Y.; Lee, H.-S.; Bae, J.-H.; Kim, M.-H.; Choi, Y. Optically Proved Molecular Alignment Behavior of Organic Semiconductor and Its Application for Organic Transistor with Enhanced Electrical Characteristics. J. Nanosci. Nanotechnol. 2017, 17, 4239–4242. [Google Scholar] [CrossRef]
- Tabi, G.D.; Nketia-Yawson, B.; Lee, J.Y.; Cho, K.; Lim, B.; Noh, Y.-Y. Fluorinated benzothiadiazole and indacenodithieno[3,2-b]thiophene based regioregular-conjugated copolymers for ambipolar organic field-effect transistors and inverters. RSC Adv. 2017, 7, 1110–1117. [Google Scholar] [CrossRef] [Green Version]
- Guillerm, V.; Weseliński, Ł.J.; Alkordi, M.; Mohideen, M.I.H.; Belmabkhout, Y.; Cairns, A.J.; Eddaoudi, M. Porous organic polymers with anchored aldehydes: A new platform for post-synthetic amine functionalization en route for enhanced CO2 adsorption properties. Chem. Commun. 2014, 50, 1937–1940. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Anil, A.G.; James, A.; Patra, A. Multifunctional Porous Organic Polymers: Tuning of Porosity, CO2, and H2 Storage and Visible-Light-Driven Photocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 27669–27678. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Pallavi, P.; Anil, A.G.; Patra, A. Fabrication of porous organic polymers in the form of powder, soluble in organic solvents and nanoparticles: A unique platform for gas adsorption and efficient chemosensing. Polym. Chem. 2015, 6, 3775–3780. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Wang, L.; Li, X.; Wang, H. Rational design on D–A conjugated P(BDT–DTBT) polymers for polymer solar cells. Polym. Chem. 2014, 5, 5200–5210. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Ma, L.; Zhan, X. Triarylamine: Versatile Platform for Organic, Dye-Sensitized, and Perovskite Solar Cells. Chem. Rev. 2016, 116, 14675–14725. [Google Scholar] [CrossRef]
- Zhang, T.; Brumboiu, I.E.; Grazioli, C.; Guarnaccio, A.; Coreno, M.; de Simone, M.; Santagata, A.; Rensmo, H.; Brena, B.; Lanzilotto, V.; et al. Lone-Pair Delocalization Effects within Electron Donor Molecules: The Case of Triphenylamine and Its Thiophene-Analog. J. Phys. Chem. C 2018, 122, 17706–17717. [Google Scholar] [CrossRef]
- Chen, C.-K.; Hsieh, H.-C.; Shih, C.-C.; Wu, C.-H.; Fu, M.-C.; Higashihara, T.; Jeng, R.-J.; Chen, W.-C. Enhancing performance of nonvolatile transistor memories via electron-accepting composition in triphenylamine-based random copolymers. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1113–1121. [Google Scholar] [CrossRef]
- Feldblyum, J.I.; McCreery, C.H.; Andrews, S.C.; Kurosawa, T.; Santos, E.J.G.; Duong, V.; Fang, L.; Ayzner, A.L.; Bao, Z. Few-layer, large-area, 2D covalent organic framework semiconductor thin films. Chem. Commun. 2015, 51, 13894–13897. [Google Scholar] [CrossRef]
- Sun, M.; Li, J.; Li, B.; Fu, Y.; Bo, Z. Toward High Molecular Weight Triphenylamine-Based Hyperbranched Polymers. Macromolecules 2005, 38, 2651–2658. [Google Scholar] [CrossRef]
- Opoku, H.; Bathula, C.; Mamo, M.D.; Shrestha, N.K.; Lee, T.; Noh, Y.-Y. Acceptor Unit Effects for Ambipolar Organic Field-Effect Transistors Based on TIPS-Benzodithiophene Copolymers. Macromol. Res. 2019, 27, 90–95. [Google Scholar] [CrossRef]
- Veres, J.; Ogier, S.D.; Leeming, S.W.; Cupertino, D.C.; Mohialdin Khaffaf, S. Low-k Insulators as the Choice of Dielectrics in Organic Field-Effect Transistors. Adv. Funct. Mater. 2003, 13, 199–204. [Google Scholar] [CrossRef]







| Polymer | Mn(KDa)/PDI a | Td (°C) b | λmax, Sol; Edge (nm) c | λmax, Film; Edge (nm) c | EHOMO (eV) d | ELUMO (eV) e | Egopt (eV) f |
|---|---|---|---|---|---|---|---|
| PTPABDT | 11.7/1.92 | 398 | 635, 730 | 640, 734 | −5.29 | −3.60 | 1.69 |
| Polymer | T (°C) | µmax (cm2V−1s−1) | µavg (cm2V−1s−1) | VT (V) | Ion/off | SS (Vdec−1) |
|---|---|---|---|---|---|---|
| TPABDT | Pristine | 2.08 × 10−4 | (1.24 ± 0.592) × 10−4 | −16.03 ± 2.05 | 7.62 × 100 | −8.92 ± 1.17 |
| 100 | 1.22 × 10−3 | (1.05 ± 0.125) × 10−3 | −24.18 ± 0.50 | 7.47 × 102 | −13.41 ± 0.92 | |
| 150 | 1.02 × 10−3 | (7.41 ± 1.75) × 10−4 | −26.37 ± 1.53 | 4.67 ×102 | −15.22 ± 0.49 | |
| 200 | 7.28 × 10−4 | (5.70 × 1.04) × 10−4 | −30.13 ± 1.51 | 4.35 × 102 | −16.29 ± 0.77 |
| Polymer | µmax (cm2V−1s−1) | Ion/off | Reference |
|---|---|---|---|
| PI(DAC-6FDA) | 8.54 × 10−2 | 2.2 × 105 | [27] |
| PI(TPA-6FDA) | 1.48 × 10−3 | 5 × 102 | [27] |
| Poly TB | 3.0 × 10−6 | 850 | [28] |
| PTTA2 | 4.86 × 10−4 | 1.24 × 102 | [31] |
| PTPABDT | 1.22 × 10−3 | 7.47 × 102 | This work |
| Bias Potential (mV) | Rs (Ω) | Rct1 (Ω) | Rct2 (Ω) | χ2 (× 10−4) |
|---|---|---|---|---|
| 10 | 1.57 | 9.22 | 446 | 7.43 |
| 50 | 1.49 | 9.69 | 1003 | 15.9 |
| 100 | 1.48 | 9.51 | 1361 | 11.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bathula, C.; Appiagyei, A.B.; Yadav, H.; K., A.K.; Ramesh, S.; Shrestha, N.K.; Shinde, S.; Kim, H.-S.; Kim, H.S.; Reddy, L.V.; et al. Facile Synthesis of Triphenylamine Based Hyperbranched Polymer for Organic Field Effect Transistors. Nanomaterials 2019, 9, 1787. https://doi.org/10.3390/nano9121787
Bathula C, Appiagyei AB, Yadav H, K. AK, Ramesh S, Shrestha NK, Shinde S, Kim H-S, Kim HS, Reddy LV, et al. Facile Synthesis of Triphenylamine Based Hyperbranched Polymer for Organic Field Effect Transistors. Nanomaterials. 2019; 9(12):1787. https://doi.org/10.3390/nano9121787
Chicago/Turabian StyleBathula, Chinna, Alfred Bekoe Appiagyei, Hemraj Yadav, Ashok Kumar K., Sivalingam Ramesh, Nabeen K. Shrestha, Surendra Shinde, Hyun-Seok Kim, Heung Soo Kim, Lebaka Veeranjaneya Reddy, and et al. 2019. "Facile Synthesis of Triphenylamine Based Hyperbranched Polymer for Organic Field Effect Transistors" Nanomaterials 9, no. 12: 1787. https://doi.org/10.3390/nano9121787