Purification of Fluorescently Derivatized N-Glycans by Magnetic Iron Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Method
2.2. Characterization Techniques
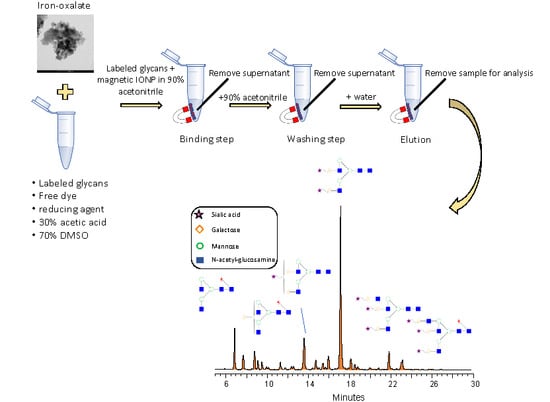
2.3. Glycan Sample Preparation
2.4. Clean-Up Optimization
2.5. LC-FLR Analysis
3. Results
3.1. Magnetic Nanoparticle-Based Glycan Purification
3.2. Characterization of PEG1000 Modified Iron-Oxalate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shubhakar, A.; Reiding, K.R.; Gardner, R.A.; Spencer, D.I.R.; Fernandes, D.L.; Wuhrer, M. High-Throughput Analysis and Automation for Glycomics Studies. Chromatographia 2015, 78, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Gornik, O.; Lauc, G. Glycosylation of serum proteins in inflammatory diseases. Dis. Markers 2008, 25, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540. [Google Scholar] [CrossRef] [PubMed]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G glycosylation in aging and diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Bondt, A.; Nicolardi, S.; Jansen, B.C.; Stavenhagen, K.; Blank, D.; Kammeijer, G.S.M.; Kozak, R.P.; Fernandes, D.L.; Hensbergen, P.J.; Hazes, J.M.W.; et al. Longitudinal monitoring of immunoglobulin A glycosylation during pregnancy by simultaneous MALDI-FTICR-MS analysis of N- and O-glycopeptides. Sci. Rep. 2016, 6, 27955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, A.; Kasela, S.; Carnero-Montoro, E.; van Iterson, M.; Štambuk, J.; Sharma, S.; van den Akker, E.; Klaric, L.; Benedetti, E.; Razdorov, G.; et al. IgG glycosylation and DNA methylation are interconnected with smoking. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Mariño, K.; Bones, J.; Kattla, J.J.; Rudd, P.M. A systematic approach to protein glycosylation analysis: A path through the maze. Nat. Chem. Biol. 2010, 6, 713. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Huhn, C.; Waterreus, W.J.; de Boer, A.R.; Neususs, C.; Hokke, C.H.; Deelder, A.M.; Wuhrer, M. Hydrophilic interaction chromatography-based high-throughput sample preparation method for N-glycan analysis from total human plasma glycoproteins. Anal. Chem. 2008, 80, 6119–6126. [Google Scholar] [CrossRef] [PubMed]
- Ruhaak, L.R.; Zauner, G.; Huhn, C.; Bruggink, C.; Deelder, A.M.; Wuhrer, M. Glycan labeling strategies and their use in identification and quantification. Anal. Bioanal. Chem. 2010, 397, 3457–3481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Váradi, C.; Lew, C.; Guttman, A. Rapid Magnetic Bead Based Sample Preparation for Automated and High Throughput N-Glycan Analysis of Therapeutic Antibodies. Anal. Chem. 2014, 86, 5682–5687. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.-S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Lin, M.; Huang, J.; Zhou, C.; Tian, W.; Yu, H.; Jiang, X.; Ye, J.; Shi, Y.; Xiao, Y.; et al. The Recent Advances of Magnetic Nanoparticles in Medicine. J. Nanomater. 2018, 2018, 8. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. Biotech 2018, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Liang, Y.; Shen, L.; Tian, S.; Zhang, K.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Maltose-Functionalized Hydrophilic Magnetic Nanoparticles with Polymer Brushes for Highly Selective Enrichment of N-Linked Glycopeptides. ACS Omega 2018, 3, 1572–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Q.; Macher, T.; Xu, Y.; Bao, Y.; Cassady, C.J. MALDI MS in-source decay of glycans using a glutathione-capped iron oxide nanoparticle matrix. Anal. Chem. 2014, 86, 8496–8503. [Google Scholar] [CrossRef] [PubMed]
- Mittermayr, S.; Albrecht, S.; Varadi, C.; Millan-Martin, S.; Bones, J. Stable Isotope Quantitative N-Glycan Analysis by Liquid Separation Techniques and Mass Spectrometry. Methods Mol. Boil. (Clifton N.J.) 2017, 1606, 353–366. [Google Scholar] [CrossRef]
- Zauner, G.; Deelder, A.M.; Wuhrer, M. Recent advances in hydrophilic interaction liquid chromatography (HILIC) for structural glycomics. Electrophoresis 2011, 32, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J.F. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Merte, L.R.; Bechstein, R.; Peng, G.; Rieboldt, F.; Farberow, C.A.; Zeuthen, H.; Knudsen, J.; Lægsgaard, E.; Wendt, S.; Mavrikakis, M.; et al. Water clustering on nanostructured iron oxide films. Nat. Commun. 2014, 5, 4193. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Váradi, C.; Sikora, E.; Vanyorek, L.; Viskolcz, B. Purification of Fluorescently Derivatized N-Glycans by Magnetic Iron Nanoparticles. Nanomaterials 2019, 9, 1480. https://doi.org/10.3390/nano9101480
Váradi C, Sikora E, Vanyorek L, Viskolcz B. Purification of Fluorescently Derivatized N-Glycans by Magnetic Iron Nanoparticles. Nanomaterials. 2019; 9(10):1480. https://doi.org/10.3390/nano9101480
Chicago/Turabian StyleVáradi, Csaba, Emőke Sikora, László Vanyorek, and Béla Viskolcz. 2019. "Purification of Fluorescently Derivatized N-Glycans by Magnetic Iron Nanoparticles" Nanomaterials 9, no. 10: 1480. https://doi.org/10.3390/nano9101480