Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
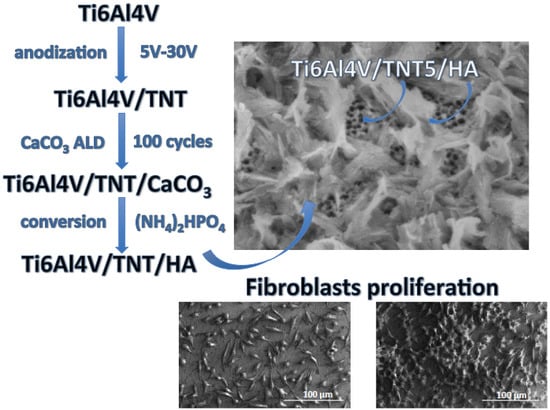
2.1. Fabrication of Ti6Al4V/TNT/HA Composite Coatings
2.1.1. Anodic Oxidation of Ti6Al4V Substrates and Structural and Morphological Studies of the Ti6Al4V/TNT System
2.1.2. Preparation of Ti6Al4V/TNT/HA Coatings and Their Structural Characterization
2.2. Wettability and the Surface Free Energy Measurements
2.3. Topography and the Mechanical Properties of Studied Systems
2.3.1. Atomic Force Microscopy
2.3.2. Mechanical Studies—Nanoindentation and Nano Scratch—Test
2.4. Biocompatibility Assays
2.4.1. Cell Culture
2.4.2. Biointegration Studies of Studied Systems
2.4.3. Statistical Analysis in the MTT Assay
2.5. Antibacterial Test
2.5.1. Bacterial Strains and Biofilm Formation on Studied Systems
2.5.2. The Assessment of S. aureus Biofilm on the Studied Systems
2.5.3. Statistical Analysis
3. Results
3.1. The Structure and Morphology of Ti6Al4V/TNT/HA
3.2. The Mechanical Properties of the Studied Systems
3.3. The Evaluation of Biointegration Properties of Ti6Al4V/TNT/HA
3.4. The Evaluation of Antibacterial Properties of Ti6Al4V/TNT/HA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Ahmadi, S.; Riahi, Z.; Eslami, A.; Sadrnezhaad, S.K. Fabrication mechanism of nanostructured HA/TNTs biomedical coatings: An improvement in nanomechanical and in vitro biological responses. J. Mater. Sci. Mater. Med. 2016, 27, 150. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Mohammadi, I.; Sadrnezhaad, S.K. Hydroxyapatite based and anodic Titania nanotube biocomposite coatings: Fabrication, characterization and electrochemical behavior. Surf. Coat. Technol. 2016, 287, 67–75. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, X.; Tian, A.; Wang, L.; Liu, C. Electrochemical properties of Ti3+ doped Ag-Ti nanotube arrays coated with hydroxyapatite. Appl. Surf. Sci. 2018, 436, 579–584. [Google Scholar] [CrossRef]
- Piszczek, P.; Lewandowska, Ż.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Szubka, M.; Talik, E.; Fiori, F. Biocompatibility of Titania Nanotube Coatings Enriched with Silver Nanograins by Chemical Vapor Deposition. Nanomaterials 2017, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Szubka, M.; Talik, E.; Nielsen, L.P.; Piszczek, P. The bioactivity and photocatalytic properties of titania nanotube coatings produced with the use of the low-potential anodization of Ti6Al4V alloy surface. Nanomaterials 2017, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Topolski, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Piszczek, P. Bioactivity Studies on Titania Coatings and the Estimation of Their Usefulness in the Modification of Implant Surfaces. Nanomaterials 2017, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Cotruta, C.M.; Vladescub, A.; Dinuc, M.; Vranceanua, D.M. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Chen, S.H.; Ho, S.C.; Chang, C.H.; Chen, C.C.; Say, W.C. Influence of roughness on invivo properties of titanium implant surface and their electrochemical behawior. Surf. Coat. Technol. 2016, 302, 215–226. [Google Scholar] [CrossRef]
- Khodaei, M.; Valanezhad, A.; Watanabe, I.; Yousefi, R. Surface and mechanical properties of modified porous titanium scaffold. Surf. Coat. Technol. 2017, 313, 61–66. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Peón, E.; Chicardi, E.; Pérez, H.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; García-Couce, J.; Galván, J.C.; García-Moreno, F.; Torresa, Y. Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Dai-Hua, H.; Wang, P.; Liu, P.; Liu, X.K.; Ma, F.C.; Zhao, J. HA coating fabricated by electrochemical deposition on modified Ti6Al4V alloy. Surf. Coat. Technol. 2016, 301, 6–12. [Google Scholar] [CrossRef]
- Lin, K.; Chang, J. Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (Hap) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–19. [Google Scholar] [CrossRef]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Kumari, R.; Majumdar, J.D. Microstructure and surface mechanical properties of plasma spray deposited and post spray heat treated hydroxyapatite (HA) based composite coating on titanium alloy (Ti-6Al-4V) substrate. Mater. Charact. 2017, 131, 12–20. [Google Scholar] [CrossRef]
- Avila, I.; Pantchev, K.; Holopainen, J.; Ritala, M.; Tuukkanen, J. Adhesion and mechanical properties of nanocrystalline hydroxyapatite coating obtained by conversion of atomic layer deposited calcium carbonate on titanium substrate. J. Mater. Sci. Mater. Med. 2018, 29, 111. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Morales, J.; Iafisco, M.; Delgado-López, J.M.; Sarda, S.; Drouet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Ma, G.; Liu, X. Hydroxyapatite: Hexagonal or monoclinic. Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- Holmes, R.; Bucholz, R.W.; Mooney, V. Porous hydroxyapatite as a bone-graft substitute in metaphyseal defects. A histometric study. J. Bone Jt. Surg. Br. Vol. 1986, 68, 904–911. [Google Scholar] [CrossRef]
- Cook, S.D.; Kay, J.F.; Thomas, K.A.; Jarcho, M. Interface mechanics and histology of titanium and hydroxylapatite-coated titanium for dental implant applications. Int. J. Oral Maxillofac. Implants 1987, 2, 15–22. [Google Scholar] [PubMed]
- Ozawa, S.; Kasugai, S. Evaluation of implant materials (hydroxyapatite, glass-ceramics, titanium) in rat bone marrow stromal cell culture. Biomaterials 1996, 17, 23–29. [Google Scholar] [CrossRef]
- Faig-Martí, J.; Gil-Mur, F.J. Hydroxyapatite coatings in prosthetic joints. Rev. Esp. Cir. Ortop. Traumatol. Engl. Ed. 2008, 52, 113–120. [Google Scholar] [CrossRef]
- Morks, M.F.; Kobayashi, A.; Fahim, N.F. Abrasive wear behavior of sprayed hydroxyapitite coatings by gas tunnel type plasma spraying. Wear 2007, 262, 204–209. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Tkalčec, E.; Kwokal, A.; Piljac, J. An in vitro study of Ti and Ti-alloys coated with sol–gel derived hydroxyapatite coatings. Surf. Coat. Technol. 2003, 165, 40–50. [Google Scholar] [CrossRef]
- Oskouei, R.H.; Fallahnezhad, K.; Kuppusami, S. An Investigation on the Wear Resistance and Fatigue Behaviour of Ti-6Al-4V Notched Members Coated with Hydroxyapatite Coatings. Materials 2016, 9, 111. [Google Scholar] [CrossRef]
- Lynn, A.K.; DuQuesnay, D.L. Hydroxyapatite-coated Ti-6Al-4V: Part 1: The effect of coating thickness on mechanical fatigue behaviour. Biomaterials 2002, 23, 1937–1946. [Google Scholar] [CrossRef]
- Byeon, I.S.; Hwang, I.J.; Choe, H.C.; Brantley, W.A. Electrochemically-coated hydroxyapatite films on nanotubular TiNb alloys prepared in solutions containing Ca, P, and Zn ions. Thin Solid Films 2016, 620, 132–138. [Google Scholar] [CrossRef]
- Hao, J.; Kuroda, S.; Ohya, K.; Bartakova, S.; Aoki, H.; Kasugai, S. Enhanced osteoblast and osteoclast responses to a thin film sputtered hydroxyapatite coating. J. Mater. Sci. Mater. Med. 2011, 22, 1489–1499. [Google Scholar] [CrossRef]
- Liu, L.; Bhatia, R.; Webster, T.J. Atomic layer deposition of nano-TiO2 thin films with enhanced biocompatibility and antimicrobial activity for orthopedic implants. Int. J. Nanomed. 2017, 12, 8711–8723. [Google Scholar] [CrossRef]
- Lugovskoy, S.; Weiss, D.; Tsadok, U.; Lugovskoy, A. Morphology and antimicrobial properties of hydroxyapatite–titanium oxide layers on the surface of Ti–6Al–4V alloy. Surf. Coat. Technol. 2016, 301, 80–84. [Google Scholar] [CrossRef]
- Holopainen, J.; Kauppinen, K.; Mizohata, K.; Santala, E.; Mikkola, E.; Heikkilä, M.; Kokkonen, H.; Leskelä, M.; Lehenkari, P.; Tuukkanen, J.; et al. Preparation and bioactive properties of nanocrystalline hydroxyapatite thin films obtained by conversion of atomic layer deposited calcium carbonate. Biointerphases 2014, 9, 031008. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Krukowski, S.; Laskus, A.; Jurkitewicz, M. Synthetic hydroxyapatite in pharmaceutical applications. Ceram. Int. 2016, 42, 2472–2487. [Google Scholar] [CrossRef]
- Drevet, R.; Jaber, N.B.; Fauré, J.; Tara, A.; Larbi, A.B.; Benhayoune, H. Electrophoretic deposition (EPD) of nano-hydroxyapatite coatings with improved mechanical properties on prosthetic Ti6Al4V substrates. Surf. Coat. Technol. 2016, 301, 94–99. [Google Scholar] [CrossRef]
- Lin, D.Y.; Zhao, Y.T. Preparation of Novel Hydroxyapatite/Yttria-Stabilized-Zirconia Gradient Coatings by Magnetron Sputtering. Adv. Eng. Mater. 2011, 13, B18–B24. [Google Scholar] [CrossRef]
- Heimann, R.B. On the Self-Affine Fractal Geometry of Plasma-Sprayed Surfaces. J. Therm. Spray Technol. 2011, 20, 898–908. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Lin, Z.Q. Preparation and bioactivity of micro-arc oxidized calcium phosphate coatings. Mater. Chem. Phys. 2013, 141, 842–849. [Google Scholar] [CrossRef]
- Shirdar, M.R.; Taheri, M.; Sudin, I.; Shafaght, A.; Keyvanfar, A.; Majid, M.Z.A. In situ synthesis of hydroxyapatite-grafted titanium nanotube composite. J. Exp. Nanosci. 2016, 11, 816–822. [Google Scholar] [CrossRef]
- Zheng, X.B.; Ding, C.X. Characterization of plasma-sprayed hydroxyapatite/TiO2 composite coatings. J. Therm. Spray Technol. 2000, 9, 520–525. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer Deposition: An Overview. Chem. Rev. 2009, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Abbass, M.K.; Ajeel, S.A.; Wadullah, H.M. Biocompatibility, Bioactivity and Corrosion Resistance of Stainless Steel 316L Nanocoated with TiO2 and Al2O3 by Atomic Layer Deposition Method. J. Phys. Conf. Ser. 2018, 1032, 012017. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Golovin, Y.I.; Tyurin, A.I.; Aslanyan, E.G.; Pirozhkova, T.S.; Vorob’ev, M.O. Local Physicomechanical Properties of Materials for Use in Calibration of Nanoindentation Instruments. Meas. Tech. 2016, 59, 911–915. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Mukhametkaliyev, T.M.; Tyurin, A.I.; Teresov, A.D.; Koval, N.N.; Pirozhkova, T.S.; Shuvarin, I.A.; Shuklinov, A.V.; Zhigachev, A.O.; Oehr, C.; et al. Effect of silicate doping on the structure and mechanical properties of thin nanostructured RF magnetron sputter-deposited hydroxyapatite films. Surf. Coat. Technol. 2015, 275, 176–184. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophile, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 123–148. ISBN 978-953-51-0537-4. [Google Scholar]
- Swain, S.; Rautray, T.R. Cu and Zn substituted hydroxyapatite coatings on titanium oxie anotubes formed by electrochemical methods. Innovare J. Eng. Technol. 2016, 4, 7–10. [Google Scholar]
- Kaabi Falahieh Asl, S.; Nemeth, S.; Tan, M.J. Electrophoretic deposition of hydroxyapatite coatings on AZ31 magnesium substrate for biodegradable implant applications. Prog. Cryst. Growth Charact. Mater. 2014, 60, 74–79. [Google Scholar] [CrossRef]
- Biological Evaluation for Medical Devices, Part 5, Tests for Cytotoxicity: In Vitro Methods; ISO 10 993-5, EN 30 993-5; International Organization for Standardization: Geneva, Switzerland, 1992.
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef]
- Barbour, M.E.; O’Sullivan, D.J.; Jenkinson, H.F.; Jagger, D.C. The effects of polishing methods on surface morphology, roughness and bacterial colonisation of titanium abutments. J. Mater. Sci. Mater. Med. 2007, 18, 1439–1447. [Google Scholar] [CrossRef]
- Zhou, W.; Zhong, X.; Wu, X.; Yuan, L.; Zhao, Z.; Wang, H.; Xia, Y.; Feng, Y.; He, J.; Chen, W. The effect of surface roughness and wettability of nanostructured TiO2 film on TCA-8113 epithelial-like cells. Surf. Coat. Technol. 2006, 200, 6155–6160. [Google Scholar] [CrossRef]
- Teixeira, A.I.; Abrams, G.A.; Bertics, P.J.; Murphy, C.J.; Nealey, P.F. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J. Cell Sci. 2003, 116, 1881–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicco, S.R.; Vona, D.; Gristina, R.; Sardella, E.; Ragni, R.; Lo Presti, M.; Farinola, G.M. Biosilica from Living Diatoms: Investigations on Biocompatibility of Bare and Chemically Modified Thalassiosira weissflogii Silica Shells. Bioengineering 2016, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, K.B.; Jeon, H.S.; Park, H.K. Effects of surface nano-topography on human osteoblast filopodia. Anal. Sci. 2011, 27, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.; Trujillo, N.A.; Popat, K.C. Comparative cell behavior on titania nanotubes filled with HAP. In Proceedings of the Society for Biomaterials 2014 Annual Meeting, Denver, CO, USA, 16–19 April 2014. [Google Scholar]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawad, P. Hydroxyapatite electrodeposition on anodized titanium nanotubes for orthopedic applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, L.; Shi, M.; Zhang, Y.; Wang, Q. Ti-GO-Ag nanocomposite: The effect of content level on the antimicrobial activity and cytotoxicity. Int. J. Nanomed. 2017, 12, 4209–4224. [Google Scholar] [CrossRef] [PubMed]
- Kucharíková, S.; Gerits, E.; De Brucker, K.; Braem, A.; Ceh, K.; Majdič, G.; Španič, T.; Pogorevc, E.; Verstraeten, N.; Tournu, H.; et al. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J. Antimicrob. Chemother. 2015, 71, 936–945. [Google Scholar] [CrossRef]
- Lan, M.-Y.; Liu, C.-P.; Huang, H.-H.; Lee, S.-W. Both enhanced biocompatibility and antibacterial activity in Ag-decorated TiO2 nanotubes. PLoS ONE 2013, 8, e75364. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef]
- Martinez-Perez, M.; Perez-Jorge, C.; Lozano, D. Evaluation of bacterial adherence of clinical isolates of Staphylococcus sp. using a competitive model. Bone Jt. Res. 2017, 6, 315–322. [Google Scholar]
- Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B.; Więckowska-Szakiel, M.; Talik, E.; Mäkelä, M.; Leskelä, M.; Piszczek, P. Optimization of the silver nanoparticles PEALD process on the surface of 1-D titania coatings. Nanomaterials 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, Ż.; Piszczek, P.; Radtke, A.; Jędrzejewski, T.; Kozak, W.; Sadowska, B. The evaluation of the impact of titania nanotube covers morphology and crystal phase on their biological properties. J. Mater. Sci. 2015, 26, 163. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Jin, B.; Qi, Y.-C.; Jiang, Q.-Y.; Gao, X.-F. Carboxylated chitosan/silver-hydroxyapatite hybrid microspheres with improved antibacterial activity and cytocompatibility. Mater. Sci. Eng. C 2017, 78, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Spengler, C.; Thewes, N.; Nolle, F.; Faidt, T.; Umanskaya, N.; Hannig, M.; Bischoff, M.; Jacobs, K. Enhanced adhesion of Streptococcus mutans to hydroxyapatite after exposure to saliva. J. Mol. Recognit. 2017, 30, e2615. [Google Scholar] [CrossRef] [PubMed]
- Valotteau, C.; Prystopiuk, V.; Pietrocola, G.; Rindi, S.; Peterle, D.; De Filippis, V.; Foster, T.J.; Speziale, P.; Dufrêne, Y.F. Single-cell and single-molecule analysis unravels the multifunctionality of the Staphylococcus aureus collagen-binding protein Cna. ACS Nano 2017, 11, 2160–2170. [Google Scholar] [CrossRef] [PubMed]











| Biomaterial Sample | Ca/P Ratio |
|---|---|
| Ti6Al4V/HA | 1.65 ± 0.06 |
| Ti6Al4V/TNT5/HA | 1.62 ± 0.01 |
| Ti6Al4V/TNT10/HA | 1.59 ± 0.04 |
| Ti6Al4V/TNT12/HA | 1.47 ± 0.04 |
| Ti6Al4V/TNT15/HA | 1.54 ± 0.02 |
| Ti6Al4V/TNT18/HA | 1.45 ± 0.06 |
| Ti6Al4V/TNT30/HA | 1.54 ± 0.04 |
| Sample | Average Contact Angle [°] ± Standard Deviation | SFE [mJ/m2] | |
|---|---|---|---|
| Measuring Liquid | |||
| Water | Diiodomethane | ||
| Ti6Al4V | 108.3 ± 0.1 | 37 ± 0.2 | 45.4 ± 0.1 |
| Ti6Al4V/HA | 57.4 ± 1.8 | 54.5 ± 2.6 | 44.6 ± 0.9 |
| Ti6Al4V/TNT5/HA | 32.2 ± 1.0 | <10 | 65.8 ± 0.2 |
| Ti6Al4V/TNT10/HA | 23.3 ± 1.8 | <10 | 69.6 ± 0.3 |
| Ti6Al4V/TNT12/HA | 22.2 ± 0.5 | <10 | 70.5 ± 0.7 |
| Ti6Al4V/TNT15/HA | 18.9 ± 1.9 | <10 | 71.6 ± 0.4 |
| Ti6Al4V/TNT18/HA | 18.2 ± 1.1 | <10 | 71.3 ± 0.2 |
| Ti6Al4V/TNT30/HA | 16.5 ± 1.3 | <10 | 71.8 ± 0.2 |
| Sample | Sa Parameter [µm] |
|---|---|
| Ti6Al4V | 0.027 |
| Ti6Al4V/HA | 0.021 |
| Ti6Al4V/TNT5/HA | 0.135 |
| Ti6Al4V/TNT10/HA | 0.113 |
| Ti6Al4V/TNT12/HA | 0.066 |
| Ti6Al4V/TNT15/HA | 0.075 |
| Ti6Al4V/TNT20/HA | 0.090 |
| Ti6Al4V/TNT30/HA | 0.078 |
| Sample | Hardness [GPa] | Young’s Modulus [GPa] | Maximum Depth of Indentation [nm] |
|---|---|---|---|
| Ti6Al4V | 16.29 ± 3.07 | 283.1 ± 30.9 | 161.1 ± 15.3 |
| Ti6Al4V/HA | 16.47 ± 2.38 | 293.5 ± 30.8 | 158.4 ± 9.7 |
| Ti6Al4V/TNT5/HA | 10.26 ± 4.08 | 256.3 ± 82.0 | 205.4 ± 46.5 |
| Ti6Al4V/TNT10/HA | 7.51 ± 1.40 | 126.1 ± 14.5 | 246.3 ± 20.1 |
| Ti6Al4V/TNT12/HA | 7.66 ± 1.70 | 248.3 ± 53.0 | 226.5 ± 32.6 |
| Ti6Al4V/TNT15/HA | 12.53 ± 3.30 | 291.6 ± 47.1 | 179.3 ± 23.5 |
| Ti6Al4V/TNT20/HA | 10.53 ± 2.59 | 160.8 ± 33.2 | 210.6 ± 31.5 |
| Ti6Al4V/TNT30/HA | 7.51 ± 1.48 | 239.9 ± 38.4 | 226.2 ± 22.4 |
| Nano Scratch Test Properties | ||
|---|---|---|
| Sample | Critical Friction [mN] | Critical Load [mN] |
| Ti6Al4V/HA | 282.1 ± 52.8 | 229.0 ± 37.9 |
| Ti6Al4V/TNT5/HA | 165.6 ± 28.2 | 166.9 ± 21.7 |
| Ti6Al4V/TNT10/HA | 153.6 ± 39.6 | 139.2 ± 26.3 |
| Ti6Al4V/TNT12/HA | 124.0 ± 13.0 | 128.9 ± 13.0 |
| Ti6Al4V/TNT15/HA | 202.6 ± 45.0 | 175.5 ± 31.9 |
| Ti6Al4V/TNT20/HA | 149.3 ± 6.5 | 160.4 ± 13.4 |
| Ti6Al4V/TNT30/HA | 228.1 ± 54.7 | 204.1 ± 32.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radtke, A.; Ehlert, M.; Jędrzejewski, T.; Sadowska, B.; Więckowska-Szakiel, M.; Holopainen, J.; Ritala, M.; Leskelä, M.; Bartmański, M.; Szkodo, M.; et al. Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties. Nanomaterials 2019, 9, 123. https://doi.org/10.3390/nano9010123
Radtke A, Ehlert M, Jędrzejewski T, Sadowska B, Więckowska-Szakiel M, Holopainen J, Ritala M, Leskelä M, Bartmański M, Szkodo M, et al. Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties. Nanomaterials. 2019; 9(1):123. https://doi.org/10.3390/nano9010123
Chicago/Turabian StyleRadtke, Aleksandra, Michalina Ehlert, Tomasz Jędrzejewski, Beata Sadowska, Marzena Więckowska-Szakiel, Jani Holopainen, Mikko Ritala, Markku Leskelä, Michał Bartmański, Marek Szkodo, and et al. 2019. "Titania Nanotubes/Hydroxyapatite Nanocomposites Produced with the Use of the Atomic Layer Deposition Technique: Estimation of Bioactivity and Nanomechanical Properties" Nanomaterials 9, no. 1: 123. https://doi.org/10.3390/nano9010123