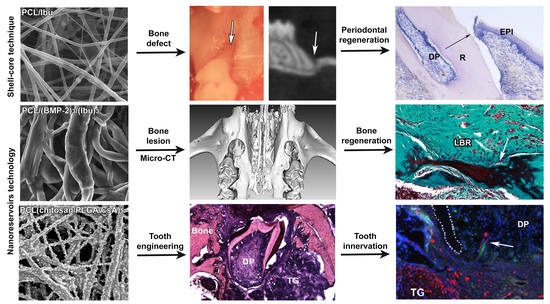
Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Cyclosporine A (CsA) Loaded PLGA Nanoparticles
2.3. PCL Scaffold Synthesis and Functionalization
2.4. In Vivo Micro-Surgical Protocols
2.5. Histology and Indirect Immunofluorescence
3. Results
3.1. Characterization of the Biomembrane
3.2. Assessment of PCL Membrane Functionalized with Ibuprofen on Periodontal Wound Healing in Periodontitis-Induced Mouse Model
3.3. Assessment of PCL Membrane Functionalized with Ibuprofen on Periodontal Wound Healing in a Mesial Bone Defect Model
3.4. Maxillary Bone Regeneration Based on Nanoreservoirs Functionalized PCL with BMP-2 and BMP-2/Ibu
3.5. Molar Bioengineering and Innervation After Bone Implantation Using CsA Functionalized Membrane
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, H.; Peres, K.G.; Peres, M.A. Retention of Teeth and Oral Health-Related Quality of Life. J. Dent. Res. 2016, 95, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Christiansen, A.L.; Cortellini, P. Vertical subclassification predicts survival of molars with class II furcation involvement during supportive periodontal care. J. Clin. Periodontol. 2017, 44, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Buti, J.; Pini Prato, G.; Tonetti, M.S. Periodontal regeneration compared with access flap surgery in human intra-bony defects 20-year follow-up of a randomized clinical trial: Tooth retention, periodontitis recurrence and costs. J. Clin. Periodontol. 2017, 44, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Agossa, K.; Morand, D.N.; Tenenbaum, H.; Davideau, J.L.; Huck, O. Systemic Application of Anti-inflammatory Agents in Periodontal Treatment. Clin. Anti-Inflamm. Anti-Allergy Drugs 2015, 2. [Google Scholar] [CrossRef]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000 2015, 67, 131–186. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Lang, N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol 2000 2013, 62, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Karring, T.; Nyman, S.; Gottlow, J.; Laurell, L. Development of the biological concept of guided tissue regeneration—Animal and human studies. Periodontol 2000 1993, 1, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cabezas, R.; Davideau, J.L.; Tenenbaum, H.; Huck, O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2016, 43, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Noordijk, M.; Davideau, J.L.; Eap, S.; Huck, O.; Fioretti, F.; Stoltz, J.F.; Bacon, W.; Benkirane-Jessel, N.; Clauss, F. Bone defects and future regenerative nanomedicine approach using stem cells in the mutant Tabby mouse model. Biomed. Mater. Eng. 2015, 25 (Suppl. 1), 111–119. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L.; Gunsolley, J.C. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects: A systematic review. Ann. Periodontol. 2003, 8, 227–265. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; McCulloch, C.A.; Narayanan, A.S.; Pitaru, S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000 2000, 24, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Morand, D.N.; Davideau, J.L.; Clauss, F.; Jessel, N.; Tenenbaum, H.; Huck, O. Cytokines during periodontal wound healing: Potential application for new therapeutic approach. Oral Dis. 2017, 23, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Strub, M.; Van Bellinghen, X.; Fioretti, F.; Bornert, F.; Benkirane-Jessel, N.; Idoux-Gillet, Y.; Kuchler-Bopp, S.; Clauss, F. Maxillary Bone Regeneration Based on Nanoreservoirs Functionalized ε-Polycaprolactone Biomembranes in a Mouse Model of Jaw Bone Lesion. BioMed Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Morand, D.N.; Thomas, L.; Bugueno, I.M.; Aragon, J.; Irusta, S.; Keller, L.; Benkirane-Jessel, N.; Tenenbaum, H.; Huck, O. Synthesis of a Novel Electrospun Polycaprolactone Scaffold Functionalized with Ibuprofen for Periodontal Regeneration: An In Vitro and In Vivo Study. Materials 2018, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Guzalinuer, A.; Muhetaer, H.; Wu, H.; Paerhati, A. Experimental study on the transforming growth factor β3 combined with dental pulp stem cells in early bone integration of implant. Zhonghua Kou Qiang Yi Xue Za Zhi 2018, 53, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, C.; Donati, M.; Cecchinato, D.; Szathvary, I.; Corrà, E.; Lindhe, J. Effect of socket grafting with deproteinized bone mineral: An RCT on dimensional alterations after 6 months. Clin. Oral Implants Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Sapata, V.M.; Hämmerle, C.H.F.; Wu, H.; Hu, X.L.; Lin, Y. Combined use of xenogeneic bone substitute material covered with a native bilayer collagen membrane for alveolar ridge preservation: A randomized controlled clinical trial. Clin. Oral Implants Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Colangeli, W.; Riccelli, U.; Giudice, A.; Barca, I.; Caruso, D.; Novembre, D.; Tortosa, C.; Cordaro, R.; Cristofaro, M.G. Jaw bones regeneration using mesenchymal stem cells: A single-center experience. Ann. Ital. Chir. 2018, 89, 20–23. [Google Scholar] [PubMed]
- Peng, L.; Ye, L.; Zhou, X.D. Mesenchymal stem cells and tooth engineering. Int. J. Oral Sci. 2009, 1, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Kuchler-Bopp, S.; Mendoza, S.A.; Poliard, A.; Lesot, H. Tooth engineering: Searching for dental mesenchymal cells sources. Front. Physiol. 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Nadiri, A.; Bopp-Kuchler, S.; Perrin-Schmitt, F.; Lesot, H. Dental epithelial histomorphogenesis in vitro. J. Dent. Res. 2005, 84, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Nait Lechguer, A.; Kuchler-Bopp, S.; Hu, B.; Haïkel, Y.; Lesot, H. Vascularization of engineered teeth. J. Dent. Res. 2008, 87, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Bécavin, T.; Keller, L.; Kökten, T.; Fioretti, F.; Weickert, J.L.; Deveaux, E.; Benkirane-Jessel, N.; Kuchler-Bopp, S. Nanofibers implant functionalized by neural growth factor as a strategy to innervate a bioengineered tooth. Adv. Healthc. Mater. 2014, 3, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Kökten, T.; Bécavin, T.; Keller, L.; Weickert, J.L.; Kuchler-Bopp, S.; Lesot, H. Immunomodulation stimulates the innervation of engineered tooth organ. PLoS ONE 2014, 9, e86011. [Google Scholar] [CrossRef] [Green Version]
- Roozbehi, A.; Joghataie, M.T.; Mehdizadeh, M.; Mirzaei, A.; Delaviz, H. The effects of cyclosporin-A on functional outcome and axonal regrowth following spinal cord injury in adult rats. Acta Med. Iran. 2012, 50, 226–232. [Google Scholar] [PubMed]
- Kuchler-Bopp, S.; Larrea, A.; Petry, L.; Idoux-Gillet, Y.; Sebastian, V.; Ferrandon, A.; Schwinté, P.; Arruebo, M.; Benkirane-Jessel, N. Promoting bioengineered tooth innervation using nanostructured and hybrid scaffolds. Acta Biomater. 2017, 50, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Palomares, C.; Ferrand, A.; Facca, S.; Fioretti, F.; Ladam, G.; Kuchler-Bopp, S.; Regnier, T.; Mainard, D.; Benkirane-Jessel, N. Smart hybrid materials equipped by nanoreservoirs of therapeutics. ACS Nano 2012, 6, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Saadi-Thiers, K.; Huck, O.; Simonis, P.; Tilly, P.; Fabre, J.E.; Tenenbaum, H.; Davideau, J.L. Periodontal and systemic responses in various mice models of experimental periodontitis: Respective roles of inflammation duration and Porphyromonas gingivalis infection. J. Periodontol. 2013, 84, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V. Membranes for Periodontal Regeneration-A Materials Perspective. Front. Oral Biol. 2015, 17, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Jian, C.; Xu, F.; Bao, T.; Lan, S.; Wu, G.; Qi, B.; Bai, Z.; Yu, A. Vancomycin-impregnated electrospun polycaprolactone (PCL) membrane for the treatment of infected bone defects: An animal study. J. Biomater. Appl. 2018, 32, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Beachley, V.; Wen, X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Prog. Polym. Sci. 2010, 35, 868–892. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fawzi-Grancher, S.; Lavalle, P.; Frochot, C.; Viriot, M.L.; Muller, S.; Wang, X. In vitro biocompatibility of different polyester membranes. Biomed. Mater. Eng. 2006, 16 (Suppl. 4), S131–S136. [Google Scholar] [PubMed]
- Deng, M.; James, R.; Laurencin, C.T.; Kumbar, S.G. Nanostructured polymeric scaffolds for orthopaedic regenerative engineering. IEEE Trans. Nanobiosci. 2012, 11, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mély, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan based nanoreservoirs of growth factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000 2016, 71, 82–112. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.M.; Takebe, J.; Offenbacher, S.; Cooper, L.F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 2002, 30, 26–31. [Google Scholar] [CrossRef]
- Sharma, S.; Sapkota, D.; Xue, Y.; Rajthala, S.; Yassin, M.A.; Finne-Wistrand, A.; Mustafa, K. Delivery of VEGFA in bone marrow stromal cells seeded in copolymer scaffold enhances angiogenesis, but is inadequate for osteogenesis as compared with the dual delivery of VEGFA and BMP2 in a subcutaneous mouse model. Stem Cell Res. Ther. 2018, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhen, R.; Yang, J.; Wang, Y.; Li, Y.; Chen, B.; Song, Y.; Ma, G.; Yang, B. Hepatocyte growth factor improves bone regeneration via the bone morphogenetic protein-2-mediated NF-κB signaling pathway. Mol. Med. Rep. 2018, 17, 6045–6053. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, Y.; Chen, L.; Mo, Y.; Huang, P.; Ma, R. Guided bone regeneration using a bone tissue engineering complex consisting of a poly-dl-lactide membrane and bone mesenchymal stem cells. Oncotarget 2017, 9, 16380–16388. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zheng, X.; Zhang, W.; Chen, M.; Wang, Z.; Hao, C.; Huang, J.; Yuan, Z.; Zhang, Y.; Wang, M.; et al. Mesenchymal stem cells in oriented PLGA/ACECM composite scaffolds enhance structure-specific regeneration of hyaline cartilage in a rabbit model. Stem Cells Int. 2018, 2018, 6542198. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.L.; Kapila, Y.; Lin, G.H. Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, T.; Lin, S.; Shi, S.; Lin, Y. Nanomaterials for Craniofacial and Dental Tissue Engineering. J. Dent. Res. 2017, 96, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Caicco, M.J.; Cooke, M.J.; Wang, Y.; Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. A hydrogel composite system for sustained epi-cortical delivery of Cyclosporin A to the brain for treatment of stroke. J. Control. Release 2013, 166, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Pini Prato, G.P.; Tonetti, M.S. No detrimental effect of fibrin glue on the regeneration of intrabony defects. A controlled clinical trial. J. Clin. Periodontol. 1995, 22, 697–702. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications. Nanomaterials 2018, 8, 337. https://doi.org/10.3390/nano8050337
Batool F, Strub M, Petit C, Bugueno IM, Bornert F, Clauss F, Huck O, Kuchler-Bopp S, Benkirane-Jessel N. Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications. Nanomaterials. 2018; 8(5):337. https://doi.org/10.3390/nano8050337
Chicago/Turabian StyleBatool, Fareeha, Marion Strub, Catherine Petit, Isaac Maximiliano Bugueno, Fabien Bornert, François Clauss, Olivier Huck, Sabine Kuchler-Bopp, and Nadia Benkirane-Jessel. 2018. "Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications" Nanomaterials 8, no. 5: 337. https://doi.org/10.3390/nano8050337