Highly Efficient Red Cabbage Anthocyanin Inserted TiO2 Aerogel Nanocomposites for Photocatalytic Reduction of Cr(VI) under Visible Light
Abstract
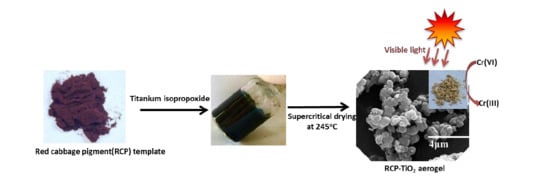
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterizations
2.3. Photocatalytic Activity
3. Results
3.1. Characterizations
3.2. Photocatalytic Tests
3.3. Effect of pH on the Photocatalytic Reduction of Cr(VI)
3.4. Effect of Initial Chromium Concentration on the Photocatalytic Reduction of Cr(VI) by RCP-AG(15)
3.5. Recyclability and Stability of Photocatalysts
3.6. Possible Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Yang, Y.; Fan, R.; Jiang, Z. Co-sensitized dye-sensitized solar cells based on d10 coordinate complexes towards their optoelectronic properties. New J. Chem. 2010, 34, 2599. [Google Scholar] [CrossRef]
- Kumari, A.; Mondal, I.; Pal, U. A simple carbazole based sensitizer attached to a nafion-coated-TiO2 photocatalyst: The impact of controlling parameters towards visible light driven h2 production. New J. Chem. 2015, 39, 713–720. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Wongcharee, K.; Meeyoo, V.; Chavadej, S. Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol. Energy Mater. Sol. Cells 2007, 91, 566–571. [Google Scholar] [CrossRef]
- Gokilamani, N.; Muthukumarasamy, N.; Thambidurai, M.; Ranjitha, A.; Velauthapillai, D. Basella alba rubra spinach pigment-sensitized TiO2 thin film-based solar cells. Appl. Nanosci. 2014, 5, 297–303. [Google Scholar] [CrossRef]
- Calogero, G.; Barichello, J.; Citro, I.; Mariani, P.; Vesce, L.; Bartolotta, A.; Di Carlo, A.; Di Marco, G. Photoelectrochemical and spectrophotometric studies on dye-sensitized solar cells (DSCs) and stable modules (DSCMs) based on natural apocarotenoids pigments. Dyes Pigments 2018, 155, 75–83. [Google Scholar] [CrossRef]
- Arapitsas, P.; Turner, C. Pressurized solvent extraction and monolithic column-hplc/dad analysis of anthocyanins in red cabbage. Talanta 2008, 74, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Molar absorptivity (epsilon) and spectral characteristics of cyanidin-based anthocyanins from red cabbage. Food Chem. 2016, 197, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.; Robbins, R.J.; Collins, T.M.; Kondo, T.; Yoshida, K.; Dangles, O. Red cabbage anthocyanins: The influence of d-glucose acylation by hydroxycinnamic acids on their structural transformations in acidic to mildly alkaline conditions and on the resulting color. Dyes Pigments 2018, 158, 342–352. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, T.Y.; Ko, H.S.; Han, E.M.; Lee, S.-H.; Kim, J.-H.; Lee, J.W. Analysis of chameleonic change of red cabbage depending on broad pH range for dye-sensitized solar cells. J. Nanosci. Nanotechnol. 2015, 15, 5840–5844. [Google Scholar] [CrossRef] [PubMed]
- Buraidah, M.H.; Teo, L.P.; Yusuf, S.N.F.; Noor, M.M.; Kufian, M.Z.; Careem, M.A.; Majid, S.R.; Taha, R.M.; Arof, A.K. TiO2/chitosan-nh4i(+i2)-bmii-based dye-sensitized solar cells with anthocyanin dyes extracted from black rice and red cabbage. Int. J. Photoenergy 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Gong, W.-J.; Tao, H.-W.; Zi, G.-L.; Yang, X.-Y.; Yan, Y.-L.; Li, B.; Wang, J.-Q. Visible light photodegradation of dyes over mesoporous titania prepared by using chrome azurol S as template. Res. Chem. Intermed. 2009, 35, 751–760. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Sun, Q.; Wang, W.; Yan, Z.; Gong, W.; Min, L. Uv and solar light degradation of dyes over mesoporous crystalline titanium dioxides prepared by using commercial synthetic dyes as templates. J. Mater. Chem. 2009, 19, 6597. [Google Scholar] [CrossRef]
- Yan, Z.; Gong, W.; Chen, Y.; Duan, D.; Li, J.; Wang, W.; Wang, J. Visible-light degradation of dyes and phenols over mesoporous titania prepared by using anthocyanin from red radish as template. Int. J. Photoenergy 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, C.; Wang, H.; Yao, Q.; Fan, B.; Chen, Y.; Sun, Q.; Jin, C.; Xu, X. A 3D titanate aerogel with cellulose as the adsorption-aggregator for highly efficient water purification. J. Mater. Chem. A 2017, 5, 5813–5819. [Google Scholar] [CrossRef]
- Wang, J.; Uma, S.; Klabunde, K.J. Visible light photocatalysis in transition metal incorporated titania-silica aerogels. Appl. Catal. B Environ. 2004, 48, 151–154. [Google Scholar] [CrossRef]
- Schwan, M.; Ratke, L. Flexibilisation of resorcinol–formaldehyde aerogels. J. Mater. Chem. A 2013, 1, 13462. [Google Scholar] [CrossRef]
- Maleki, H.; Hüsing, N. Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects. Appl. Catal. B Environ. 2018, 221, 530–555. [Google Scholar] [CrossRef]
- Kim, Y.N.; Shao, G.N.; Jeon, S.J.; Imran, S.M.; Sarawade, P.B.; Kim, H.T. Sol–gel synthesis of sodium silicate and titanium oxychloride based TiO2–SiO2 aerogels and their photocatalytic property under UV irradiation. Chem. Eng. J. 2013, 231, 502–511. [Google Scholar] [CrossRef]
- Sadrieyeh, S.; Malekfar, R. The effects of hydrolysis level on structural properties of titania aerogels. J. Non-Cryst. Solids 2017, 457, 175–179. [Google Scholar] [CrossRef]
- Alwin, S.; Sahaya Shajan, X.; Menon, R.; Nabhiraj, P.Y.; Warrier, K.G.K.; Mohan Rao, G. Surface modification of titania aerogel films by oxygen plasma treatment for enhanced dye adsorption. Thin Solid Films 2015, 595, 164–170. [Google Scholar] [CrossRef]
- Padhi, D.K.; Parida, K. Facile fabrication of α-feooh nanorod/rgo composite: A robust photocatalyst for reduction of Cr(VI) under visible light irradiation. J. Mater. Chem. A 2014, 2, 10300–10312. [Google Scholar] [CrossRef]
- Yu, J.; Zhuang, S.; Xu, X.; Zhu, W.; Feng, B.; Hu, J. Photogenerated electron reservoir in hetero-p–n cuo–zno nanocomposite device for visible-light-driven photocatalytic reduction of aqueous Cr(VI). J. Mater. Chem. A 2015, 3, 1199–1207. [Google Scholar] [CrossRef]
- Wang, C.-C.; Du, X.-D.; Li, J.; Guo, X.-X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B Environ. 2016, 193, 198–216. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H. Preparation and characterization of Ag-doped TiO2 nanomaterials and their photocatalytic reduction of Cr(VI) under visible light. Appl. Surf. Sci. 2014, 321, 396–403. [Google Scholar] [CrossRef]
- Zhong, J.S.; Wang, Q.Y.; Zhou, J.; Chen, D.Q.; Ji, Z.G. Highly efficient photoelectrocatalytic removal of rhb and Cr(VI) by cu nanoparticles sensitized TiO2 nanotube arrays. Appl. Surf. Sci. 2016, 367, 342–346. [Google Scholar] [CrossRef]
- Kumar Yadav, S.; Jeevanandam, P. Thermal decomposition approach for the synthesis of CdS–TiO2 nanocomposites and their catalytic activity towards degradation of rhodamine b and reduction of Cr(VI). Ceram. Int. 2015, 41, 2160–2179. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Pan, H.; Xu, D.; Lu, T.; Mao, L.; Meng, X.; Chen, Z.; Zhang, D.; Zhu, S. Fabrication of agbr/boron-doped reduced graphene oxide aerogels for photocatalytic removal of Cr(VI) in water. RSC Adv. 2017, 7, 36000–36006. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Deng, K.; She, Y.; Yu, Y.; Tang, H. Visible light photocatalytic reduction of cr(vi) on TiO2 in situ modified with small molecular weight organic acids. Appl. Catal. B: Environ. 2010, 95, 400–407. [Google Scholar] [CrossRef]
- Di Credico, B.; Redaelli, M.; Bellardita, M.; Calamante, M.; Cepek, C.; Cobani, E.; D’Arienzo, M.; Evangelisti, C.; Marelli, M.; Moret, M.; et al. Step-by-step growth of hkust-1 on functionalized TiO2 surface: An efficient material for CO2 capture and solar photoreduction. Catalysts 2018, 8, 353. [Google Scholar] [CrossRef]
- Zi, G.; Wang, Y.; Zheng, K.; Zhao, H.; Wang, F.; Zhang, W.; Yan, Z.; He, J.; Wang, J. Visible light photodegradation of rhodamine b over vdf/ctfe copolymer-templated crystalline mesoporous titania. Res. Chem. Intermed. 2012, 38, 2383–2391. [Google Scholar] [CrossRef]
- Pan, J.H.; Zhao, X.S.; Lee, W.I. Block copolymer-templated synthesis of highly organized mesoporous TiO2-based films and their photoelectrochemical applications. Chem. Eng. J. 2011, 170, 363–380. [Google Scholar] [CrossRef]
- Di Credico, B.; Bellobono, I.R.; D’Arienzo, M.; Fumagalli, D.; Redaelli, M.; Scotti, R.; Morazzoni, F. Efficacy of the reactive oxygen species generated by immobilized TiO2 in the photocatalytic degradation of diclofenac. Int. J. Photoenergy 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Smith, J.R.; Amaya, K.R.; Bredemeier, R.T.; Banta, S.; Cropek, D.M. Selective biomolecular photocatalytic decomposition using peptide-modified TiO2 nanoparticles. Appl. Catal. B Environ. 2015, 176–177, 315–324. [Google Scholar] [CrossRef]
- Abbasizadeh, S.; Keshtkar, A.R.; Mousavian, M.A. Preparation of a novel electrospun polyvinyl alcohol/titanium oxide nanofiber adsorbent modified with mercapto groups for uranium(vi) and thorium(iv) removal from aqueous solution. Chem. Eng. J. 2013, 220, 161–171. [Google Scholar] [CrossRef]
- Kapica-Kozar, J.; Michalkiewicz, B.; Wrobel, R.J.; Mozia, S.; Piróg, E.; Kusiak-Nejman, E.; Serafin, J.; Morawski, A.W.; Narkiewicz, U. Adsorption of carbon dioxide on tepa-modified TiO2/titanate composite nanorods. New J. Chem. 2017, 41, 7870–7885. [Google Scholar] [CrossRef]
- Araña, J.; Rodríguez, J.M.D.; Díaz, O.G.; Melián, J.A.H.; Rodríguez, C.F.; Peña, J.P. The effect of acetic acid on the photocatalytic degradation of catechol and resorcinol. Appl. Catal. A Gen. 2006, 299, 274–284. [Google Scholar] [CrossRef]
- Araña, J.; Alonso, A.P.; Rodríguez, J.M.D.; Colón, G.; Navío, J.A.; Peña, J.P. Ftir study of photocatalytic degradation of 2-propanol in gas phase with different TiO2 catalysts. Appl. Catal. B Environ. 2009, 89, 204–213. [Google Scholar] [CrossRef]
- Gokilamani, N.; Muthukumarasamy, N.; Thambidurai, M.; Ranjitha, A.; Velauthapillai, D. Utilization of natural anthocyanin pigments as photosensitizers for dye-sensitized solar cells. J. Sol-Gel Sci. Technol. 2013, 66, 212–219. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Wan Salleh, W.N.; Jaafar, J.; Rosmi, M.S.; Hir, Z.A.M.; Mutalib, M.A.; Ismail, A.F.; Tanemura, M. Carbon as amorphous shell and interstitial dopant in mesoporous rutile TiO2: Bio-template assisted sol-gel synthesis and photocatalytic activity. Appl. Surf. Sci. 2017, 393, 46–59. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Wu, D.; Sun, Y. Visible-light responsive dye-modified TiO2 photocatalyst. J. Solid State Chem. 2008, 181, 593–602. [Google Scholar] [CrossRef]
- Atashbar, M.Z.; Sun, H.T.; Gong, B.; Wlodarski, W.; Lamb, R. Xps study of nb-doped oxygen sensing TiO2 thin films prepared by sol-gel method. Thin Solid Films 1998, 326, 238–244. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Nowaczyk, G.; Jancelewicz, M.; Pavlenko, M.; Załęski, K.; Jurga, S. Structural and XPS studies of PSi/TiO2 nanocomposites prepared by ALD and Ag-assisted chemical etching. Appl. Surf. Sci. 2015, 347, 777–783. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, W.; Wang, M.; Li, S.; Chen, J.; Zhang, Y.; Cao, S. In situ synthesis of carbon-doped TiO2 single-crystal nanorods with a remarkably photocatalytic efficiency. Appl. Catal. B Environ. 2017, 209, 311–319. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, L.; Song, W.; Qin, Z.; Zeng, D.; Xie, C. In situ synthesis of c-TiO2/g-C3N4 heterojunction nanocomposite as highly visible light active photocatalyst originated from effective interfacial charge transfer. Appl. Catal. B: Environ. 2017, 202, 489–499. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, P.; Dong, Y.; Huang, M.; Liu, B. A high-surface-area mesoporous sulfated nano-titania solid superacid catalyst with exposed (101) facets for esterification: Facile preparation and catalytic performance. New J. Chem. 2014, 38, 4541. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Lu, G.; Tu, Y.; Wu, J. Improved performance of surface functionalized TiO2/activated carbon for adsorption–photocatalytic reduction of Cr(VI) in aqueous solution. Mater. Sci. Semicond. Process. 2015, 39, 362–370. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.; Wang, S.; Li, L.; Liu, X. Influence of tunable pore size on photocatalytic and photoelectrochemical performances of hierarchical porous TiO2/c nanocomposites synthesized via dual-templating. Appl. Catal. B: Environ. 2017. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Sun, H.; Lu, G.; Wu, J. The multiple roles of ethylenediamine modification at TiO2 /activated carbon in determining adsorption and visible-light-driven photoreduction of aqueous Cr(VI). J. Alloys Compd. 2016, 662, 165–172. [Google Scholar] [CrossRef]
- Marinho, B.A.; Cristóvão, R.O.; Djellabi, R.; Loureiro, J.M.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic reduction of Cr(VI) over TiO2-coated cellulose acetate monolithic structures using solar light. Appl. Catal. B: Environ. 2017, 203, 18–30. [Google Scholar] [CrossRef]
- Chen, K.-C.; Wu, J.-Y.; Liou, D.-J.; Hwang, S.-C.J. Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol. 2003, 101, 57–68. [Google Scholar] [CrossRef]
- Zielińska, B.; Grzechulska, J.; Grzmil, B.; Morawski, A.W. Photocatalytic degradation of reactive black 5. Appl. Catal. B Environ. 2001, 35, L1–L7. [Google Scholar] [CrossRef]
- He, J.; Wang, J.; Chen, Y.; Zhang, J.; Duan, D.; Wang, Y.; Yan, Z. A dye-sensitized pt@uio-66(zr) metal-organic framework for visible-light photocatalytic hydrogen production. Chem. Commun. (Camb.) 2014, 50, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Osman, T.A.; Toprak, M.S.; Muhammed, M.; Yilmaz, E.; Uheida, A. Visible light photocatalytic reduction of Cr(VI) by surface modified cnt/titanium dioxide composites nanofibers. J. Mol. Catal. A: Chem. 2016, 424, 45–53. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.; Liu, L.; Zong, R.; Yao, W.; Liang, Y.; Zhu, Y. Removal of Cr(VI) by 3D TiO2-graphene hydrogel via adsorption enriched with photocatalytic reduction. Appl. Catal. B Environ. 2016, 199, 412–423. [Google Scholar] [CrossRef]
- Han, J.; Zhu, G.; Hojamberdiev, M.; Peng, J.; Zhang, X.; Liu, Y.; Ge, B.; Liu, P. Rapid adsorption and photocatalytic activity for rhodamine b and Cr(VI) by ultrathin bioi nanosheets with highly exposed {001} facets. New J. Chem. 2015, 39, 1874–1882. [Google Scholar] [CrossRef]
- Zhang, H.K.; Lu, H.; Wang, J.; Zhou, J.T.; Sui, M. Cr(VI) reduction and Cr(III) immobilization by Acinetobacter sp. Hk-1 with the assistance of a novel quinone/graphene oxide composite. Environ. Sci. Technol. 2014, 48, 12876–12885. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, C.; Gao, F.; Mailhot, G.; Pan, G. Algae decorated TiO2/Ag hybrid nanofiber membrane with enhanced photocatalytic activity for Cr(VI) removal under visible light. Chem. Eng. J. 2017, 314, 622–630. [Google Scholar] [CrossRef]
- Liu, F.; Yu, J.; Tu, G.; Qu, L.; Xiao, J.; Liu, Y.; Wang, L.; Lei, J.; Zhang, J. Carbon nitride coupled Ti-SBA15 catalyst for visible-light-driven photocatalytic reduction of Cr(VI) and the synergistic oxidation of phenol. Appl. Catal. B: Environ. 2017, 201, 1–11. [Google Scholar] [CrossRef]
- Park, S.; Kim, W.; Selvaraj, R.; Kim, Y. Spontaneous reduction of Cr(VI) using insns 2 under dark condition. Chem. Eng. J. 2017, 321, 97–104. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Saccomanni, A.; Selli, E. Cr(VI) photocatalytic reduction: Effects of simultaneous organics oxidation and of gold nanoparticles photodeposition on TiO2. J. Hazard. Mater. 2012, 211–212, 188–195. [Google Scholar] [CrossRef] [PubMed]














| Samples | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Eg (eV) |
|---|---|---|---|---|
| AG | 101 | 0.6 | 16 | 3.24 |
| S-AG | 100 | 0.5 | 16 | - |
| RCP-AG(6) | 122 | 0.5 | 15 | 2.68 |
| RCP-AG(9) | 149 | 0.3 | 7 | 2.61 |
| RCP-AG(12) | 202 | 0.5 | 8 | 2.49 |
| RCP-AG(15) | 141 | 0.3 | 8 | 2.36 |
| RCP-AG(18) | 164 | 0.4 | 7 | 2.49 |
| Samples | Ti 2p (%) | O1s (%) | C1s (%) |
|---|---|---|---|
| AG | 20.5 | 52.2 | 21.3 |
| RCP-AG (15) | 20.9 | 49.0 | 27.5 |
| Catalyst | Adsorption Yield after 1 h (%) | Adsorption Yield after 3 h (%) |
|---|---|---|
| AG | 10.5 | 11.0 |
| S-AG | 27.8 | 28.5 |
| RCP-AG(6) | 30.2 | 30.1 |
| RCP-AG(9) | 20.9 | 21.2 |
| RCP-AG(12) | 32.8 | 33.4 |
| RCP-AG(15) | 19.1 | 20.1 |
| RCP-AG(18) | 32.5 | 32.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Jiang, L.; Li, Y.; Li, G.; Yang, Y.; He, J.; Wang, J.; Yan, Z. Highly Efficient Red Cabbage Anthocyanin Inserted TiO2 Aerogel Nanocomposites for Photocatalytic Reduction of Cr(VI) under Visible Light. Nanomaterials 2018, 8, 937. https://doi.org/10.3390/nano8110937
Yang H, Jiang L, Li Y, Li G, Yang Y, He J, Wang J, Yan Z. Highly Efficient Red Cabbage Anthocyanin Inserted TiO2 Aerogel Nanocomposites for Photocatalytic Reduction of Cr(VI) under Visible Light. Nanomaterials. 2018; 8(11):937. https://doi.org/10.3390/nano8110937
Chicago/Turabian StyleYang, Haiyan, Liang Jiang, Yizhou Li, Guoqing Li, Yepeng Yang, Jiao He, Jiaqiang Wang, and Zhiying Yan. 2018. "Highly Efficient Red Cabbage Anthocyanin Inserted TiO2 Aerogel Nanocomposites for Photocatalytic Reduction of Cr(VI) under Visible Light" Nanomaterials 8, no. 11: 937. https://doi.org/10.3390/nano8110937