Anchoring Gated Mesoporous Silica Particles to Ethylene Vinyl Alcohol Films for Smart Packaging Applications
Abstract
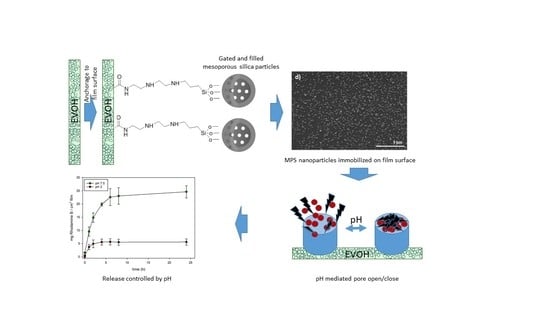
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Mesoporous Silica Particless
2.3. Mesoporous Silica Particle Loading and Functionalization
2.4. Characterization of Mesoporous Silica Particles
2.5. Preparation and Functionalization of EVOH Films
2.6. N3-MSP Deposition
2.7. Surface Analysis
2.8. Controlled Release from the Films
3. Results and Discussion
3.1. MSP Preparation and Characterization
3.2. EVOH Film Surface Analysis
3.3. MSP Immobilization
3.4. Controlled Release Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Larson, A.M.; Klibanov, A.M. Biocidal packaging for pharmaceuticals, foods, and other perishables. Annu. Rev. Chem. Biomol. Eng. 2013, 4, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Yam, K.L.; Lee, D.S. Emerging Food Packaging Technologies: Principles and Practice; Woodhead Publishing: Sawston, UK, 2012. [Google Scholar]
- Gavara, R.; López-Carballo, G.; Hernández-Muñoz, P.; Catalá, R.; Muriel-Galet, V.; Cerisuelo, J.P.; Dominguez, I. Practical Guide to Antimicrobial Active Packaging; Smithers Rapra Publishing: Surrey, UK, 2015; p. 263. [Google Scholar]
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Almenar, E.; Hernandez-Munoz, P.; Lagaron, J.M.; Catala, R.; Gavara, R. Overview of active polymer-based packaging technologies for food applications. Food Rev. Int. 2004, 20, 357–387. [Google Scholar] [CrossRef]
- Charles, F.; Sanchez, J.; Gontard, N. Absorption kinetics of oxygen and carbon dioxide scavengers as part of active modified atmosphere packaging. J. Food Eng. 2006, 72, 1–7. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Muriel-Galet, V.; Bermudez, J.M.; Aucejo, S.; Catala, R.; Gavara, R.; Hernandez-Munoz, P. Mathematical model to describe the release of an antimicrobial agent from an active package constituted by carvacrol in a hydrophilic evoh coating on a pp film. J. Food Eng. 2012, 110, 26–37. [Google Scholar] [CrossRef]
- Chalier, P.; Ben Arfa, A.; Guillard, V.; Gontard, N. Moisture and temperature triggered release of a volatile active agent from soy protein coated paper: Effect of glass transition phenomena on carvacrol diffusion coefficient. J. Agric. Food Chem. 2009, 57, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Bushman, A.C.; Castle, G.J.; Pastor, R.D.; Reighard, T.S. Process for Activiating Oxygen Scavenger Components during a Gable-Top Carton Filling Process. U.S. Patent 6,689,314B2, 10 February 2004. [Google Scholar]
- Goddard, J.M.; Talbert, J.N.; Hotchkiss, J.H. Covalent attachment of lactase to low-density polyethylene films. J. Food Sci. 2007, 72, E36–E41. [Google Scholar] [CrossRef] [PubMed]
- Muriel Galet, V.; Talbert, J.N.; Hernandez Munoz, P.; Gavara, R.; Goddard, J.M. Covalent immobilization of lysozyme on ethylene vinyl alcohol films for nonmigrating antimicrobial packaging applications. J. Agric. Food Chem. 2013, 61, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sillard, C.; Belgacem, M.N.; Bras, J. Nisin anchored cellulose nanofibers for long term antimicrobial active food packaging. RSC Adv. 2016, 6, 12437–12445. [Google Scholar] [CrossRef]
- Roman, M.J.; Decker, E.A.; Goddard, J.M. Biomimetic polyphenol coatings for antioxidant active packaging applications. Colloid Interface Sci. Commun. 2016, 13, 10–13. [Google Scholar] [CrossRef] [Green Version]
- Vasile, C.; Pâslaru, E.; Sdrobis, A.; Pricope, G.; Ioanid, G.E.; Darie, R.N. Plasma assisted functionalization of synthetic and natural polymers to obtain new bioactive food packaging materials. In Proceedings of the Application of Radiation Technology in Development of Advanced Packaging Materials for Food Products Vienna, Austria, 22–26 April 2013; Safrani, A., Ed.; pp. 100–110. [Google Scholar]
- Mihindukulasuriya, S.D.F.; Lim, L.T. Nanotechnology development in food packaging: A review. Trends Food Sci. Technol. 2014, 40, 149–167. [Google Scholar] [CrossRef]
- Cerisuelo, J.P.; Alonso, J.; Aucejo, S.; Gavara, R.; Hernandez-Munoz, P. Modifications induced by the addition of a nanoclay in the functional and active properties of an evoh film containing carvacrol for food packaging. J. Membr. Sci. 2012, 423–424, 247–256. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhang, C.; Chi, H.; Li, L.; Lan, T.; Han, P.; Chen, H.; Qin, Y. Development of antimicrobial packaging film made from poly(lactic acid) incorporating titanium dioxide and silver nanoparticles. Molecules 2017, 22, 1170. [Google Scholar] [CrossRef] [PubMed]
- Mal, N.K.; Fujiwara, M.; Tanaka, Y.; Taguchi, T.; Matsukata, M. Photo-switched storage and release of guest molecules in the pore void of coumarin-modified mcm-41. Chem. Mater. 2003, 15, 3385–3394. [Google Scholar] [CrossRef]
- Mal, N.K.; Fujiwara, M.; Tanaka, Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature 2003, 421, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Tseng, H.R.; Wong, J.W.; Stoddart, J.F.; Zink, J.I. An operational supramolecular nanovalve. J. Am. Chem. Soc. 2004, 126, 3370–3371. [Google Scholar] [CrossRef] [PubMed]
- Casasus, R.; Marcos, M.D.; Martinez-Manez, R.; Ros-Lis, J.V.; Soto, J.; Villaescusa, L.A.; Amoros, P.; Beltran, D.; Guillem, C.; Latorre, J. Toward the development of ionically controlled nanoscopic molecular gates. J. Am. Chem. Soc. 2004, 126, 8612–8613. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Martínez-Máñez, R.; Sancenón, F. Controlled release using mesoporous materials containing gate-like scaffoldings. Expert Opin. Drug Deliv. 2009, 6, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Hung, B.-Y.; Kuthati, Y.; Kankala, R.K.; Kankala, S.; Deng, J.-P.; Liu, C.-L.; Lee, C.-H. Utilization of enzyme-immobilized mesoporous silica nanocontainers (IBN-4) in prodrug-activated cancer theranostics. Nanomaterials 2015, 5, 2169–2191. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Liu, C.-G.; Chen, A.-Z.; Wang, S.-B.; Xu, P.-Y.; Mende, L.K.; Liu, C.-L.; Lee, C.-H.; Hu, Y.-F. Overcoming multidrug resistance through the synergistic effects of hierarchical pH-sensitive, ros-generating nanoreactors. ACS Biomater. Sci. Eng. 2017, 3, 2431–2442. [Google Scholar] [CrossRef]
- Huang, P.-K.; Lin, S.-X.; Tsai, M.-J.; Leong, M.K.; Lin, S.-R.; Kankala, R.K.; Lee, C.-H.; Weng, C.-F. Encapsulation of 16-hydroxycleroda-3,13-dine-16,15-olide in mesoporous silica nanoparticles as a natural dipeptidyl peptidase-4 inhibitor potentiated hypoglycemia in diabetic mice. Nanomaterials 2017, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Kuthati, Y.; Kankala, R.K.; Busa, P.; Lin, S.-X.; Deng, J.-P.; Mou, C.-Y.; Lee, C.-H. Phototherapeutic spectrum expansion through synergistic effect of mesoporous silica trio-nanohybrids against antibiotic-resistant gram-negative bacterium. J. Photochem. Photobiol. B Biol. 2017, 169, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Sancenon, F.; Pascual, L.; Oroval, M.; Aznar, E.; Martinez-Manez, R. Gated silica mesoporous materials in sensing applications. Chemistryopen 2015, 4, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Kuthati, Y.; Liu, C.-L.; Mou, C.-Y.; Lee, C.-H. Killing cancer cells by delivering a nanoreactor for inhibition of catalase and catalytically enhancing intracellular levels of ros. RSC Adv. 2015, 5, 86072–86081. [Google Scholar] [CrossRef]
- Perez-Esteve, E.; Ruiz-Rico, M.; de la Torre, C.; Villaescusa, L.A.; Sancenon, F.; Marcos, M.D.; Amoros, P.; Martinez-Manez, R.; Manuel Barat, J. Encapsulation of folic acid in different silica porous supports: A comparative study. Food Chem. 2016, 196, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Raju, S.; Mohana, N.; Sampath, P.; Jayakumari, L. Effects of nanomaterials on polymer composites—An expatiate view. Rev. Adv. Mater. Sci. 2014, 38, 40–54. [Google Scholar]
- Perez-Esteve, E.; Fuentes, A.; Coll, C.; Acosta, C.; Bernardos, A.; Amoros, P.; Marcos, M.D.; Sancenon, F.; Martinez-Manez, R.; Barat, J.M. Modulation of folic acid bioaccessibility by encapsulation in pH-responsive gated mesoporous silica particles. Microporous Mesoporous Mater. 2015, 202, 124–132. [Google Scholar] [CrossRef]
- Uchida, E.; Uyama, Y.; Ikada, Y. Sorption of low-molecular-weight anions into thin polycation layers grafted onto a film. Langmuir 1993, 9, 1121–1124. [Google Scholar] [CrossRef]
- Kang, E.T.; Tan, K.L.; Kato, K.; Uyama, Y.; Ikada, Y. Surface modification and functionalization of polytetrafluoroethylene films. Macromolecules 1996, 29, 6872–6879. [Google Scholar] [CrossRef]
- Perez-Esteve, E.; Ruiz-Rico, M.; Martinez-Manez, R.; Manuel Barat, J. Mesoporous silica-based supports for the controlled and targeted release of bioactive molecules in the gastrointestinal tract. J. Food Sci. 2015, 80, E2504–E2516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, X.; Tang, J.; Yang, Y.-W. Surface immobilization of pH-responsive polymer brushes on mesoporous silica nanoparticles by enzyme mimetic catalytic atrp for controlled cargo release. Polymers 2016, 8, 277. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Stetsyshyn, Y. Stability of halloysite, imogolite, and boron nitride nanotubes in solvent media. Appl. Sci. 2018, 8, 1068. [Google Scholar] [CrossRef]
- Bryce-Smith, D.; Gilbert, A. Photodegradation of polymers. In Photochemistry; Royal Society of Chemistry: Cambridge, UK, 1993; pp. 448–458. [Google Scholar]








| Sample | BET Area (m2/g) | Pore Volume (c3/g) | Pore Size (nm) | αRh (mg/gsolid) | αN3 (mg/gsolid) | Z-Potential (mV) |
|---|---|---|---|---|---|---|
| M | 1072 | 0.91 | 2.62 | - | - | −38 |
| M-Rh-N3 | 243 | - | - | 15.3 | 81 | 41 |
| N | 986 | 0.84 | 2.51 | - | - | −36 |
| N-Rh-N3 | 143 | - | - | 17.2 | 142 | 43 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muriel-Galet, V.; Pérez-Esteve, É.; Ruiz-Rico, M.; Martínez-Máñez, R.; Barat, J.M.; Hernández-Muñoz, P.; Gavara, R. Anchoring Gated Mesoporous Silica Particles to Ethylene Vinyl Alcohol Films for Smart Packaging Applications. Nanomaterials 2018, 8, 865. https://doi.org/10.3390/nano8100865
Muriel-Galet V, Pérez-Esteve É, Ruiz-Rico M, Martínez-Máñez R, Barat JM, Hernández-Muñoz P, Gavara R. Anchoring Gated Mesoporous Silica Particles to Ethylene Vinyl Alcohol Films for Smart Packaging Applications. Nanomaterials. 2018; 8(10):865. https://doi.org/10.3390/nano8100865
Chicago/Turabian StyleMuriel-Galet, Virginia, Édgar Pérez-Esteve, María Ruiz-Rico, Ramón Martínez-Máñez, José Manuel Barat, Pilar Hernández-Muñoz, and Rafael Gavara. 2018. "Anchoring Gated Mesoporous Silica Particles to Ethylene Vinyl Alcohol Films for Smart Packaging Applications" Nanomaterials 8, no. 10: 865. https://doi.org/10.3390/nano8100865