Excipient Nanoemulsions for Improving Oral Bioavailability of Bioactives
Abstract
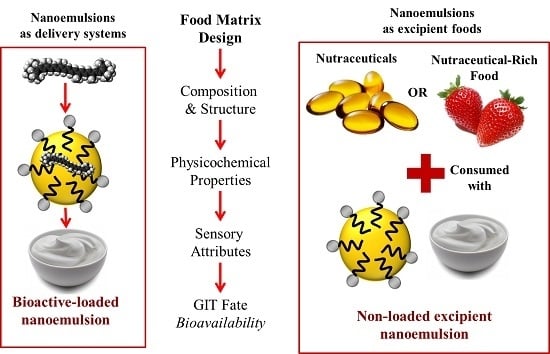
:1. Introduction
1.1. Concept of Excipient Foods
1.2. Nanoemulsions as Excipient Foods
2. Excipient Foods

| Food Source | Compatible Excipient Food | Bioactive Compound |
|---|---|---|
| Salad | Salad dressing | Carotenoids |
| Fruits and berries | Cream, ice cream, yogurt | Flavonoids, vitamins, coenzyme Q10 |
| Vegetables | Edible coatings, sauce | Carotenoids, vitamins, phytosterols/stanols |
| Nuts and seeds | Edible coatings, sauce | Flavonoids, vitamins |
| Meat | Sauce | Conjugated linoleic acids |
| Dairy products (cheese) | Sauce | Conjugated linoleic acids |
| Fish | Sauce | ω-3 fatty acids |
3. Factors Limiting the Bioavailability of Bioactive Compounds

| Bioaccessibility | Absorption | Transformation |
|---|---|---|
| Liberation: the bioactive must be released from the drug, supplement of food matrix | Mucus layer: the bioactive must be transported across the mucus layer that coats epithelium cells | Chemical transformation: some bioactives undergo chemical degradation within the GIT |
| Solubilization: the bioactive must be solubilized within GIT fluids | Tight junctions: some bioactives may pass through tight junctions separating epithelium cells | Biochemical transformation: some bioactives are digested or metabolized by enzymes in the GIT |
| Interactions: the bioactive may interact with other food components | Membrane permeation: some bioactives may be transported through the phospholipid bilayer | |
| Active Transport: some bioactives are transported by active transport proteins | ||
| Efflux: some bioactives are removed from epithelium cells by efflux proteins |
4. Nanoemulsions
4.1. Formation
4.2. Characteristics
4.2.1. Droplet Size

4.2.2. Droplet Composition
4.2.3. Interfacial Properties
4.3. Mechanisms of Action
4.3.1. Bioaccessibility
Changes in the Bioactive Release from the Food Matrix
Solubilization in the Intestinal Fluids
4.3.2. Absorption

Increase in Mucus Layer Transport
Increase in the Permeability of Cell Membranes
Efflux Inhibition
4.3.3. Transformation
4.4. Examples of Excipient Nanoemulsions
| Emulsion | Nutraceuticals | Excipient Emulsion Influencing Nutraceuticals Bioaccessibility | Reference |
|---|---|---|---|
| Corn oil emulsions | Curcumin in Powdered Form | The solubility and bioaccessibility of curcumin was significantly improved by incubating and co-ingesting with excipient emulsion. | [32] |
| Corn oil emulsions | Curcumin in Powdered Form | Emulsifier type and droplet size of exhibited a significant effect on the solubility and bioaccessibility of curcumin. | [66] |
| Corn oil emulsions | Curcumin in Powdered Form | The bioaccessibility of curcumin depended on oil type and concentration. | [67] |
| Corn oil, medium chain triglycerides or orange oil emulsions | Carotenoids in yellow peppers | Increased carotenoid bioacessibility from yellow peppers when consumed with corn oil nanoemulsions as excipient emulsions. | [68] |
| Olive oil emulsions | Carotenoids in Carrot and Tomato suspensions | Adding olive emulsions to carrot and tomato suspensions increased carotenoid uptake in the micellar phase. | [69] |
| Peanut oil emulsions | Carotenoids in Tomato juice | Lycopene bioaccessibility was dependent on emulsification and emulsifier type | [70] |
| Various emulsions | Carotenoids in vegetables and salads | Addition of oil to salads and vegetables increased lycopene bioaccessibility depending on fatty acid type | [71,72] |
4.4.1. Increase in Bioaccessibility
4.4.2. Changes in Absorption and Transformation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moelants, K.R.N.; Lemmens, L.; Vandebroeck, M.; van Buggenhout, S.; van Loey, A.M.; Hendrickx, M.E. Relation between Particle Size and Carotenoid Bioaccessibility in Carrot- and Tomato-Derived Suspensions. J. Agric. Food Chem. 2012, 60, 11995–12003. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W.; Porter, C.J. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, D.; Li, C.; Zhou, Y.; Pao, L.H.; Karim, A. Drug, meal and formulation interactions influencing drug absorption after oral administration—Clinical implications. Clin. Pharmacokinet. 1999, 36, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.N.; Amidon, G.L. A mechanistic approach to understanding the factors affecting drug absorption: A review of fundamentals. J. Clin. Pharmacol. 2002, 42, 620–643. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A Metabolism: An Update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, E.; Carvajal-Lerida, I.; Jaren-Galan, M.; Garrido-Fernandez, J.; Perez-Galvez, A.; Hornero-Mendez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L. Challenges and Solutions to Incorporation of Nutraceuticals in Foods. Annu. Rev. Food Sci. Technol. 2015, 6, 463–477. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, F.; Xiao, H. The Nutraceutical Bioavailability Classification Scheme: Classifying Nutraceuticals According to Factors Limiting Their Oral Bioavailability. Annu. Rev. Food Sci. Technol. 2015, 6, 299–327. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Li, Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Adv. Colloid Interface Sci. 2010, 159, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.H.; Pouton, C.W.; Cuine, J.F.; Charman, W.N. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 673–691. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Excipient foods: Designing food matrices that improve the oral bioavailability of pharmaceuticals and nutraceuticals. Food Funct. 2014, 5, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural Design Principles for Delivery of Bioactive Components in Nutraceuticals and Functional Foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
- Brown, M.J.; Ferruzzi, M.G.; Nguyen, M.L.; Cooper, D.A.; Eldridge, A.L.; Schwartz, S.J.; White, W.S. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am. J. Clin. Nutr. 2004, 80, 396–403. [Google Scholar] [PubMed]
- McClements, D.J. Utilizing food effects to overcome challenges in delivery of lipophilic bioactives: Structural design of medical and functional foods. Expert Opin. Drug Deliv. 2013, 10, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, R.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Hayat, K.; Karangwa, E.; Bashari, M.; Zhang, X.M. An Overview of Ultrasound-Assisted Food-Grade Nanoemulsions. Food Eng. Rev. 2013, 5, 139–157. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Impact of microfluidization or ultrasound processing on the antimicrobial activity against Escherichia coli of lemongrass oil-loaded nanoemulsions. Food Control 2014, 37, 292–297. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; McClements, D.J. Influence of Nanoemulsion Addition on the Stability of Conventional Emulsions. Food Biophys. 2015. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Potential biological fate of ingested nanoemulsions: Influence of particle characteristics. Food Funct. 2012, 3, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; McClements, D.J. New Mathematical Model for Interpreting pH-Stat Digestion Profiles: Impact of Lipid Droplet Characteristics on in Vitro Digestibility. J. Agric. Food Chem. 2010, 58, 8085–8092. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Modulating β-carotene bioaccessibility by controlling oil composition and concentration in edible nanoemulsions. Food Chem. 2013, 139, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Couedelo, L.; Amara, S.; Lecomte, M.; Meugnier, E.; Monteil, J.; Fonseca, L.; Pineau, G.; Cansell, M.; Carriere, F.; Michalski, M.C.; et al. Impact of various emulsifiers on ALA bioavailability and chylomicron synthesis through changes in gastrointestinal lipolysis. Food Funct. 2015, 6, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Low, D.Y.; D’Arcy, B.; Gidley, M.J. Mastication effects on carotenoid bioaccessibility from mango fruit tissue. Food Res. Int. 2015, 67, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.Q.; Liu, W.; Liu, C.M.; Xiao, H.; McClements, D.J. Utilizing Food Matrix Effects To Enhance Nutraceutical Bioavailability: Increase of Curcumin Bioaccessibility Using Excipient Emulsions. J. Agric. Food Chem. 2015, 63, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Kalantzi, L.; Goumas, K.; Kalioras, V.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 2006, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N.; Porter, C.J.H.; Mithani, S.; Dressman, J.B. Physicochemical and physiological mechanisms for the effects of food on drug absorption: The role of lipids and pH. J. Pharm. Sci. 1997, 86, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Varum, F.J.O.; Hatton, G.B.; Basit, A.W. Food, physiology and drug delivery. Int. J. Pharm. 2013, 457, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Kossena, G.A.; Charman, W.N.; Wilson, C.G.; O’Mahony, B.; Lindsay, B.; Hempenstall, J.M.; Davison, C.L.; Crowley, P.J.; Porter, C.J.H. Low dose lipid formulations: Effects on gastric emptying and biliary secretion. Pharm. Res. 2007, 24, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Verrijssen, T.A.J.; Smeets, K.H.G.; Christiaens, S.; Palmers, S.; van Loey, A.M.; Hendrickx, M.E. Relation between in vitro lipid digestion and β-carotene bioaccessibility in β-carotene-enriched emulsions with different concentrations of L-α-phosphatidylcholine. Food Res. Int. 2015, 67, 60–66. [Google Scholar] [CrossRef]
- Cho, H.T.; Salvia-Trujillo, L.; Kim, J.; Park, Y.; Xiao, H.; McClements, D.J. Droplet size and composition of nutraceutical nanoemulsions influences bioavailability of long chain fatty acids and Coenzyme Q10. Food Chem. 2014, 156, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.H.; Charman, W.N. Intestinal lymphatic drug transport: An update. Adv. Drug Deliv. Rev. 2001, 50, 61–80. [Google Scholar] [CrossRef]
- Lafitte, G.; Thuresson, K.; Soderman, O. Diffusion of nutrients molecules and model drug carriers through mucin layer investigated by magnetic resonance imaging with chemical shift resolution. J. Pharm. Sci. 2007, 96, 258–263. [Google Scholar] [CrossRef] [PubMed]
- des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.J.; Preat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Bohdanowicz, M.; Grinstein, S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 2013, 93, 69–106. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Brayden, D.J.; Feighery, L.; McClean, S. Cracking the junction: Update on the progress of gastrointestinal absorption enhancement in the delivery of poorly absorbed drugs. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 117–168. [Google Scholar] [CrossRef]
- Buyukozturk, F.; Benneyan, J.C.; Carrier, R.L. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J. Control. Release 2010, 142, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Hwang, B.H.; Doshi, N.; Mitragotri, S. A permeation enhancer for increasing transport of therapeutic macromolecules across the intestine. J. Control. Release 2013, 172, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Pillay, V.; Hibbins, A.R.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Ndesendo, V.M.K. Orally Administered Therapeutic Peptide Delivery: Enhanced Absorption Through the Small Intestine Using Permeation Enhancers. Int. J. Pept. Res. Ther. 2012, 18, 259–280. [Google Scholar] [CrossRef]
- Wang, X.X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Mullin, J.M. Zinc Supplementation Modifies Tight Junctions and Alters Barrier Function of CACO-2 Human Intestinal Epithelial Layers. Dig. Dis. Sci. 2013, 58, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, H.J.R.; Hamman, J.H. Paracellular drug absorption enhancement through tight junction modulation. Expert Opin. Drug Deliv. 2013, 10, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Dudhatra, G.B.; Mody, S.K.; Awale, M.M.; Patel, H.B.; Modi, C.M.; Kumar, A.; Kamani, D.R.; Chauhan, B.N. A Comprehensive Review on Pharmacotherapeutics of Herbal Bioenhancers. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Katsumi, H.; Kusamori, K.; Sakane, T. Improvement of Intestinal Absorption of Poorly Absorbable Drugs by Various Sugar Esters. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2014, 134, 47–53. [Google Scholar] [CrossRef]
- Jiang, L.F.; Long, X.W.; Meng, Q. Rhamnolipids enhance epithelial permeability in CACO-2 monolayers. Int. J. Pharm. 2013, 446, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.; Pillay, V.; Ndesendo, V.M.K.; du Toit, L.C.; Choonara, Y.E. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm. Drug Dispos. 2011, 32, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, P.P.; Wasan, K.M. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: In vitro/in vivo case studies. J. Pharm. Sci. 2007, 96, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Choi, B.C.; Kang, K.W. Effect of resveratrol on the pharmacokinetics of oral and intravenous nicardipine in rats: Possible role of P-glycoprotein inhibition by resveratrol. Pharmazie 2009, 64, 49–52. [Google Scholar] [PubMed]
- Kang, M.J.; Cho, J.Y.; Shim, B.H.; Kim, D.K.; Lee, J. Bioavailability enhancing activities of natural compounds from medicinal plants. J. Med. Plants Res. 2009, 3, 1204–1211. [Google Scholar]
- Challa, V.R.; Babu, P.R.; Challa, S.R.; Johnson, B.; Maheswari, C. Pharmacokinetic interaction study between quercetin and valsartan in rats and in vitro models. Drug Dev. Ind. Pharm. 2013, 39, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.J.; Han, H.K. Effect of Piperine, a Major Component of Black Pepper, on the Intestinal Absorption of Fexofenadine and Its Implication on Food-Drug Interaction. J. Food Sci. 2010, 75, H93–H96. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Lumpkin, J.L.; Schwartz, S.J.; Failla, M. Digestive stability, micellarization, and uptake of β-carotene isomers by CACO-2 human intestinal cells. J. Agric. Food Chem. 2006, 54, 2780–2785. [Google Scholar] [CrossRef] [PubMed]
- Boileau, A.C.; Merchen, N.R.; Wasson, K.; Atkinson, C.A.; Erdman, J.W. Cis-lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. J. Nutr. 1999, 129, 1176–1181. [Google Scholar] [PubMed]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Sanchez, B.; Tavazzi, I.; Lambelet, P.; Bortlik, K.; Williamson, G. Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 2010, 103, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Zou, L.Q.; Zheng, B.J.; Liu, W.; Liu, C.M.; Xiao, H.; McClements, D.J. Enhancing nutraceutical bioavailability using excipient emulsions: Influence of lipid droplet size on solubility and bioaccessibility of powdered curcumin. J. Funct. Foods 2015, 15, 72–83. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Liu, X.; Bi, J.F.; Xiao, H.; McClements, D.J. Increasing Carotenoid Bioaccessibility from Yellow Peppers Using Excipient Emulsions: Impact of Lipid Type and Thermal Processing. J. Agric. Food Chem. 2015, 63, 8534–8543. [Google Scholar] [CrossRef] [PubMed]
- Moelants, K.R.N.; Cardinaels, R.; Jolie, R.P.; Verrijssen, T.A.J.; van Buggenhout, S.; Zumalacarregui, L.M.; van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Relation Between Particle Properties and Rheological Characteristics of Carrot-Derived Suspensions. Food Bioprocess Technol. 2013, 6, 1127–1143. [Google Scholar] [CrossRef]
- Degrou, A.; Georgé, S.; Renard, C.M.G.C.; Page, D. Physicochemical parameters that influence carotenoids bioaccessibility from a tomato juice. Food Chem. 2013, 136, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Ferruzzi, M.G.; Schwartz, S.J.; Failla, M.L. Impact of fatty acyl composition and quantity of triglycerides on bloaccessibility of dietary carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Kotake-Nara, E.; Hase, M. Effects of Fats and Oils on the Bioaccessibility of Carotenoids and Vitamin E in Vegetables. Biosci. Biotechnol. Biochem. 2013, 77, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.-S.; Lee, S.J.; Wen, J.; Oey, I.; Ko, S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Improving the Oral Bioavailability of Curcumin Using Novel Organogel-Based Nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.F.; Chen, J.J.; Zheng, J.K.; Song, M.Y.; McClements, D.J.; Xiao, H. Enhanced lymphatic transport of bioactive lipids: Cell culture study of polymethoxyflavone incorporation into chylomicrons. Food Funct. 2013, 4, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.F.; Xiao, H.; McClements, D.J. Delivery of Lipophilic Bioactives: Assembly, Disassembly, and Reassembly of Lipid Nanoparticles. Annu. Rev. Food Sci. Technol. 2014, 5, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Aspenstrom-Fagerlund, B.; Tallkvist, J.; Ilback, N.G.; Glynn, A.W. Oleic acid decreases BCRP mediated efflux of mitoxantrone in CACO-2 cell monolayers. Food Chem. Toxicol. 2012, 50, 3635–3645. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. EDTA and α-tocopherol improve the chemical stability of astaxanthin loaded into nanostructured lipid carriers. Eur. J. Lipid Sci. Technol. 2014, 116, 968–977. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvia-Trujillo, L.; Martín-Belloso, O.; McClements, D.J. Excipient Nanoemulsions for Improving Oral Bioavailability of Bioactives. Nanomaterials 2016, 6, 17. https://doi.org/10.3390/nano6010017
Salvia-Trujillo L, Martín-Belloso O, McClements DJ. Excipient Nanoemulsions for Improving Oral Bioavailability of Bioactives. Nanomaterials. 2016; 6(1):17. https://doi.org/10.3390/nano6010017
Chicago/Turabian StyleSalvia-Trujillo, Laura, Olga Martín-Belloso, and David Julian McClements. 2016. "Excipient Nanoemulsions for Improving Oral Bioavailability of Bioactives" Nanomaterials 6, no. 1: 17. https://doi.org/10.3390/nano6010017