A Strategy for Hydroxide Exclusion in Nanocrystalline Solid-State Metathesis Products
Abstract
:1. Introduction
2. Experimental Section

| Precursors | Product | Calc. ZC ΔG0 (kJ/mol) | Calc. HZ ΔG0 (kJ/mol) |
|---|---|---|---|
| at 25 °C | at 25 °C | ||
| ZnCl2 + NaHCO3 | none | −64 | −278 |
| ZnCl2 + Na2CO3 | HZ | −86 | −391 |
| Zn(NO3)2·6H2O + Na2CO3 | HZ | −71 | −483 |
| Zn(NO3)2·6H2O + NaHCO3 | ZC | −85 | −203 |
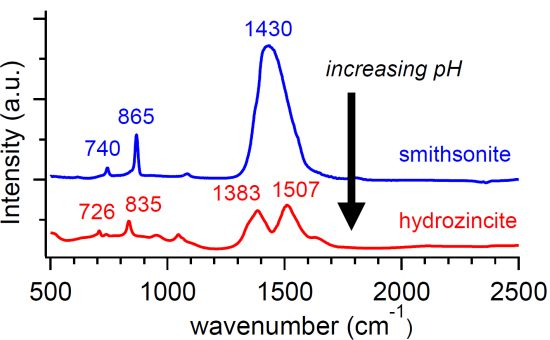
3. Results and Discussion



4. Conclusions
Acknowledgements
References
- Wiley, J.B.; Kaner, R.B. Rapid solid-state precursor synthesis of materials. Science 1992, 255, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Q.; Wang, J.S.; Wu, X.C.; Zhang, G.S.; Wei, J.Y.; Zhang, S.Q.; Li, H.; Chen, D.R. Novel method for high-yield synthesis of rutile SnO2 nanorods by oriented aggregation. Cryst. Growth Des. 2006, 6, 1584–1587. [Google Scholar] [CrossRef]
- Perera, S.; Zelenski, N.; Gillan, E.G. Synthesis of nanocrystalline TiO2 and reduced titanium oxides via rapid and exothermic metathesis reactions. Chem. Mater. 2006, 18, 2381–2388. [Google Scholar] [CrossRef]
- Lu, J.; Ng, K.M.; Yang, S. Efficient, one-step mechanochemical process for the synthesis of ZnO nanoparticles. Ind. Eng. Chem. Res. 2008, 47, 1095–1101. [Google Scholar] [CrossRef]
- Sinha, A.K.; Pradhan, M.; Sarkar, S.; Pal, T. Large-scale solid-state synthesis of Sn-SnO2 nanoparticles from layered SnO by sunlight: A material for dye degradation in water by photocatalytic reaction. Environ. Sci. Technol. 2013, 47, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.R.; Jia, D.Z.; Yu, J.Q.; Xin, X.Q.; Xue, Z. One-step solid-state reactions at ambient temperatures: A novel approach to nanocrystal synthesis. Adv. Mater. 1999, 11, 941–942. [Google Scholar] [CrossRef]
- Toberer, E.S.; Weaver, J.C.; Ramesha, K.; Seshadri, R. Macroporous monoliths of functional perovskite materials through assisted metathesis. Chem. Mater. 2004, 16, 2194–2200. [Google Scholar] [CrossRef]
- Ren, L.; Wu, Q.; Yang, C.; Zhu, L.; Li, C.; Zhang, P.; Zhang, H.; Meng, X.; Xiao, F.S. Solvent-free synthesis of zeolites from solid raw materials. J. Am. Chem. Soc. 2012, 134, 15173–15176. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, Q. Preparation of nanocrystalline Zinc carbonate and Zinc oxide via solid-state rection at room temperature. Mater. Lett. 2006, 60, 2791–2794. [Google Scholar] [CrossRef]
- Zhang, S.; Fortier, H.; Dahn, J.R. Characterization of Zinc carbonate hydroxides synthesized by precipitation from Zinc acetate and potassium carbonate solutions. Mater. Res. Bull. 2004, 39, 1939–1948. [Google Scholar] [CrossRef]
- Dean, J. Lange’s Handbook of Chemistry; McGraw Hill Book Co.: New York, NY, USA, 1985. [Google Scholar]
- Powder Diffraction File. Numbers 08-0449 and 19-1458. In Joint Commission on Powder Diffraction Standards–International Centre for Diffraction Data, Newtown Square, PA, USA,, 2003.
- Hales, M.C.; Frost, R.L. Synthesis and vibrational spectroscopic characterisation of synthetic hydrozincite and smithsonite. Polyhedron 2007, 26, 4955–4962. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, M.; Smith, D. Catalogue of 45 reference Raman spectra of minerals concerning research in art history or archaeology, especially on corroded metals and coloured glass. Spectrochim. Acta A 2003, 59, 2247–2266. [Google Scholar] [CrossRef]
- Su, B.; Li, M.; Shi, Z.; Lu, Q. From superhydrophilic to superhydrophobic: Controlling wettability of hydroxide Zinc carbonate film on Zinc plates. Langmuir 2009, 25, 3640–3645. [Google Scholar] [CrossRef] [PubMed]
- Hales, M.C.; Frost, R.L. Thermal analysis of smithsonite and hydrozincite. J. Therm. Anal. Calorim. 2008, 91, 855–860. [Google Scholar] [CrossRef]
- Koga, N.; Goshi, Y.; Yamada, S.; Pérez-Maqueda, L. Kinetic approach to partially overlapped thermal decomposition processes. J. Therm. Anal. Calorim. 2013, 111, 1463–1474. [Google Scholar] [CrossRef]
- Micković, Z.; Alexander, D.T.L.; Sienkiewicz, A.; Mionić, M.; Forró, L.; Magrez, A. Synthesis of nanosized Mn-doped ZnO by low temperature decomposition of hydrozincite precursors. Cryst. Growth Des. 2010, 10, 4437–4441. [Google Scholar]
- Zhang, J.; Wang, S.; Xu, M.; Wang, Y.; Zhu, B.; Zhang, S.; Huang, W.; Wu, S. Hierarchically porous ZnO architectures for gas sensor application. Cryst. Growth Des. 2009, 9, 3532–3537. [Google Scholar] [CrossRef]
- Nistor, S.V.; Nistor, L.C.; Stefan, M.; Ghica, D.; Aldica, G.; Barascu, J.N. Crystallization of disordered nanosized ZnO formed by thermal decomposition of nanocrystalline hydrozincite. Cryst. Growth Des. 2011, 11, 5030–5038. [Google Scholar] [CrossRef]
- Qu, X.; Jia, D. Synthesis of octahedral ZnO mesoscale superstructures via thermal decomposing octahedral Zinc hydroxide precursors. J. Cryst. Growth 2009, 311, 1223–1228. [Google Scholar] [CrossRef]
- Shamsipur, M.; Pourmortazavi, S.M.; Hajimirsadeghi, S.S.; Zahedi, M.M.; Rahimi-Nasrabadi, M. Facile synthesis of Zinc carbonate and Zinc oxide nanoparticles via direct carbonation and thermal decomposition. Ceram. Inter. 2013, 39, 819–827. [Google Scholar] [CrossRef]
- Li, Z.; Shen, X.; Feng, X.; Wang, P.; Wu, Z. Non-isothermal kinetics studies on the thermal decomposition of Zinc hydroxide carbonate. Thermochim. Acta 2005, 438, 102–106. [Google Scholar] [CrossRef]
- Alwan, A.K.; Williams, P.A. Mineral formation from aqueous solution. Part I. The deposition of hydrozincite, Zn5(OH)6(CO3)2, from natural waters. Transit. Met. Chem. 1979, 4, 128–132. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cheng, J.; Poduska, K.M. A Strategy for Hydroxide Exclusion in Nanocrystalline Solid-State Metathesis Products. Nanomaterials 2013, 3, 317-324. https://doi.org/10.3390/nano3030317
Cheng J, Poduska KM. A Strategy for Hydroxide Exclusion in Nanocrystalline Solid-State Metathesis Products. Nanomaterials. 2013; 3(3):317-324. https://doi.org/10.3390/nano3030317
Chicago/Turabian StyleCheng, Jiaqi, and Kristin M. Poduska. 2013. "A Strategy for Hydroxide Exclusion in Nanocrystalline Solid-State Metathesis Products" Nanomaterials 3, no. 3: 317-324. https://doi.org/10.3390/nano3030317