Energy Decomposition Scheme for Rectangular Graphene Flakes
Abstract
:1. Introduction
2. Previous Studies
3. Computational Methodology and Data Analysis
4. Construction of the Fit
- (i)
- The fit coefficients are , , , and . The first three coefficients are identical to the coefficients obtained with the set . Such a pronounced fit stability suggests that all the employed basis functions represent some physical contributions to the energy.
- (ii)
- The energy residuals grow with increasing values of m and n. This signifies that the fitting formula cannot be extrapolated to large values of m and n without substantial loss of accuracy.
- (iii)
- The residues corresponding to and show distinct behavior compared with the rest of residues corresponding to higher values of m and/or n.
- (iv)
- The residues corresponding to and to show somewhat distinct behavior compared with the residues corresponding to higher values of m and n.
5. Results and Discussion
- (i)
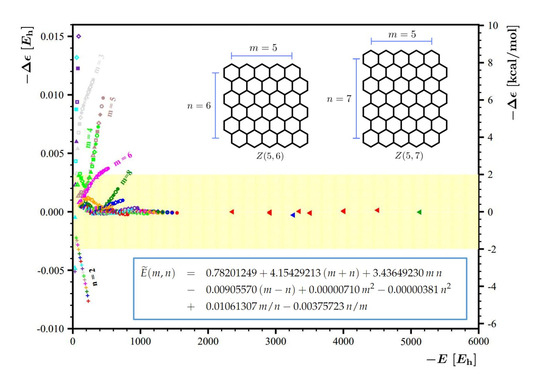
- Figure 6 and the left panel of Figure 7 clearly show that the accuracy of the constructed energy expression deteriorates when one extrapolates it outside of the fitting set. A similar effect is also expected for the best-constructed energy expression in Equation (11) when used for very large flakes with . However, as the current study shows, such a problem can be easily circumvented by adding a few new very large flakes with relevant values of m and n to the fitting set. For researchers interested in such extensions, we have included all the DFTB input files in the Supplementary Materials Section of this manuscript. The DFTB geometry optimization for the largest flake considered here, with the molecular formula C2950H152, took approximately several weeks on a 100-core computer. The reader should be aware that the optimization of a larger flake might take a considerably longer time. An interesting alternative here could be a theoretical analysis of the contributions to the total energy from the finite edge effects and possibly quantifying such an influence using non-obvious, new -dependent basis functions. Such a development is expected to improve the description of small flakes and to permit the extrapolation of the energy formula to really large values of m and n that presently escape the possibility of direct quantum chemical calculations.
- (ii)
- Small rectangular graphene flakes are known for their various interesting chemical deviations from the typical behavior of polycyclic hydrocarbons, including their open-shell ground state [53,54,55,56] and pronounced radical character [57,58,59,60,61,62,63]. Our work shows that larger flakes are more uniform, suggesting that the transition from finite, pyrene-like, small flakes to infinite, graphene-like, large flakes happens in the regime of . To investigate this issue in more detail, we have analyzed the distribution of the CC bond lengths and CCC bond angles in the transition from small to large flakes. Before discussing the results, let us only briefly comment that in the transition from small to large flakes, one would expect that the bond lengths and bond angles would become more uniform, approaching the values corresponding to an infinite graphene sheet, i.e., CC bond lengths of 1.42–1.43 Å obtained from the DFT calculations [64,65,66,67] and of 1.42 Å obtained from experiment and an angle of 120 corresponding to a hexagonal geometry. The results for square flakes defined by the formula for –20 are presented in Figure 8.These results show that the convergence to the graphene-like values is fast. In principle, the flakes already show distributions similar to . In addition to square-shaped flakes, it is interesting to check for similar convergence properties for rectangular flakes. In Figure 9, we show analogous distributions of CC bond lengths and CCC bond angles for two families of rectangular flakes, and , with values of –20. In both cases, the convergence toward the graphene-like regime is obtained faster than for the square flakes; this effect is particularly fast for the polyacene-like flakes . Despite the fast convergence to the graphene-like regime, the finite edge effects are clearly visible in all the distributions, suggesting that the hydrogen termination and edge effects constitute important local perturbations.
- (iii)
- The parameters of the fit tabulated in Table 2 show surprisingly large inertias with respect to the extension of the function set with new variables. This behavior suggests that the energy decomposition has physical meaning, and its coefficients can be interpreted as sums of various energy contributions. For a flake , the contributions can be easily identified:
- The energy of a hydrogen atom with multiplicity ;
- The energy of a carbon atom with multiplicity ;
- The energy of an aromatic ring with multiplicity ;
- The energy of a C–C bond with multiplicity ;
- The energy of a C–H bond with multiplicity .
Unfortunately, all these contributions involve only 3 fitting functions ( and ), showing that a unique determination of the five parameters , , , , and from the three coefficients , , and is not possible from our analysis. In the future studies, we plan to extend our analysis to other structured graphene flakes, including prolate rectangles [11,68], oblate rectangles [11,69], and hexagons [11,46]. We expect that the distinct dependence of the total energy on the structural parameters for these structures will help to uniquely determine the five parameters , , , , and defined above. - (iv)
- The energies used to construct the energy expression given by Equation (11) in addition to the usual size and shape dependence encoded by the parameters m and n also include contributions related to the geometry relaxation effects. In our study, all these components are treated collectively. It would be very interesting to consider the relaxation effects individually, for example, by starting the geometry optimization from a rectangular patch of an idealized infinite graphene sheet with uniform hydrogen terminations. The relaxation effects can be divided into three types of contributions: (1) those corresponding to the relaxation of the carbon sublattice, (2) those corresponding to the relaxation of the hydrogen sublattice, and (3) those corresponding to the synergic relaxation of both lattices simultaneously. Such an analysis is beyond the scope of the current study.
- (v)
- Numerous studies tried to correlate various topological invariants with the energetic stability of polycyclic aromatic hydrocarbons. The vast efforts of the graph-theoretically oriented chemists over the last few decades have created substantial literature on this topic. (Probably the best existing account describing the body of results on the Kekulé count is the monography of Cyvin and Gutman [11]; the results on other invariants are scattered throughout the literature.) Our study may show that such an effort might be somewhat superfluous as similar effects can be achieved more readily by correlating the energetics with appropriate structural parameters (and their simple algebraic functions) of the whole family of analyzed structures.
6. Conclusions
- (i)
- It has been recognized that small graphene flakes with are structurally dissimilar to larger flakes due to their finite-size effects. Those structures have been excluded from the fitting set, which finally comprised 121 energies of flakes with .
- (ii)
- It has been noted that in order to be able to extrapolate the energy expression outside of the fitting region, we need to include in the fitting set several larger structures with .
- (iii)
- The set of fitting functions resulting from structural considerations and comprising physically meaningful variables needs to be expanded to . The physical interpretation of these new variables remains to be understood.
- (iv)
- Performance tests of the energy expression have been performed using two additional graphene flakes, and .
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ML | Machine learning |
| DFTB3 | Third-order density-functional tight-binding |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| SVD | Singular value decomposition |
Appendix A. Correlations between Topological Invariants and Total Electronic Energies
Appendix A.1. Energies E(m,n) as Functions of lnK and lnC

Appendix A.2. Energy Residuals for Various Fitting Sets Involving Only Topological Invariants


References
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models, 1st ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Elstner, M.; Seifert, G. Density functional tight binding. Philos. Trans. R. Soc. 2014, 372, 20120483. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.A.; Vassilev-Galindo, V.; Cheng, B.; Chmiela, S.; Gastegger, M.; Müller, K.R.; Tkatchenko, A. Combining machine learning and computational chemistry for predictive insights into chemical systems. Chem. Rev. 2021, 121, 9816–9872. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Tkatchenko, A.; Müller, K.R.; von Lilienfeld, O.A. Fast and accurate modeling of molecular atomization energies with machine learning. Phys. Rev. Lett. 2012, 108, 058301. [Google Scholar] [CrossRef]
- Montavon, G.; Rupp, M.; Gobre, V.; Vazquez-Mayagoitia, A.; Hansen, K.; Tkatchenko, A.; Müller, K.R.; von Lilienfeld, O.A. Machine learning of molecular electronic properties in chemical compound space. New J. Phys. 2013, 15, 095003. [Google Scholar] [CrossRef]
- Schütt, K.T.; Arbabzadah, F.; Chmiela, S.; Müller, K.R.; Tkatchenko, A. Quantum-chemical insights from deep tensor neural networks. Nat. Commun. 2017, 8, 13890. [Google Scholar] [CrossRef]
- De, S.; Bartók, A.P.; Csányi, G.; Ceriotti, M. Comparing molecules and solids across structural and alchemical space. Phys. Chem. Chem. Phys. 2016, 18, 13754–13769. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, S.; Tkatchenko, A.; Sauceda, H.E.; Poltavsky, I.; Schütt, K.T.; Müller, K.R. Machine learning of accurate energy-conserving molecular force fields. Sci. Adv. 2017, 3, e1603015. [Google Scholar] [CrossRef]
- Faber, F.A.; Hutchison, L.; Huang, B.; Gilmer, J.; Schoenholz, S.S.; Dahl, G.E.; Vinyals, O.; Kearnes, S.; Riley, P.F.; von Lilienfeld, O.A. Prediction errors of molecular machine learning models lower than hybrid DFT error. J. Chem. Theory Comput. 2017, 13, 5255–5264. [Google Scholar] [CrossRef] [PubMed]
- Schütt, K.T.; Sauceda, H.E.; Kindermans, P.J.; Tkatchenko, A.; Müller, K.R. SchNet—A deep learning architecture for molecules and Materials. J. Chem. Phys. 2018, 148, 241722. [Google Scholar] [CrossRef]
- Cyvin, S.J.; Gutman, I. Kekulé Structures in Benzenoid Hydrocarbons; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar]
- Gutman, I.; Cyvin, S.J.; si Chen, R.; rong Lin, K. Number of Kekulé structures of multiple zigzag chain aromatics. Monatsh. Chem. 1993, 124, 117–125. [Google Scholar] [CrossRef]
- Weiße, A.; Fehske, H. Exact diagonalization techniques. In Computational Many-Particle Physics; Fehske, H., Schneider, R., Weiße, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 529–544. [Google Scholar]
- James, D.K.; Tour, J.M. Graphene: Powder, flakes, ribbons, and sheets. Acc. Chem. Res. 2013, 46, 2307–2318. [Google Scholar] [CrossRef] [PubMed]
- Kairi, M.I.; Dayou, S.; Kairi, N.I.; Bakar, S.A.; Vigolo, B.; Mohamed, A.R. Toward high production of graphene flakes—A review on recent developments in their synthesis methods and scalability. J. Mater. Chem. A 2018, 6, 15010–15026. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Fowler, P.W.; Manolopoulos, D.E. An Atlas of Fullerenes; Dover: Mineola, NY, USA, 2006. [Google Scholar]
- Schwerdtfeger, P.; Wirz, L.; Avery, J. Program Fullerene: A software package for constructing and analyzing structures of regular fullerenes. J. Comput. Chem. 2013, 34, 1508–1526. [Google Scholar] [CrossRef]
- Assadollahzadeh, B.; Schwerdtfeger, P. A systematic search for minimum structures of small gold clusters Aun(n=2–20) and their electronic properties. J. Chem. Phys. 2009, 131, 064306. [Google Scholar] [CrossRef] [PubMed]
- Assadollahzadeh, B.; Schäfer, S.; Schwerdtfeger, P. Electronic properties for small tin clusters Snn(n≤20) from density functional theory and the convergence toward the solid state. J. Comput. Chem. 2010, 31, 929–937. [Google Scholar] [CrossRef]
- Assadollahzadeh, B.; Thierfelder, C.; Schwerdtfeger, P. From clusters to the solid state: Global minimum structures for cesium clusters Csn(n=2–20,∞) and their electronic properties. Phys. Rev. B 2008, 78, 245423. [Google Scholar] [CrossRef]
- Firouzi, R.; Zahedi, M. Polyacenes electronic properties and their dependence on molecular size. J. Mol. Struct. Theochem 2008, 862, 7–15. [Google Scholar] [CrossRef]
- Li, W.; Irle, S.; Witek, H.A. Convergence in the evolution of nanodiamond Raman spectra with particle size: A theoretical investigation. ACS Nano 2010, 4, 4475–4486. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.; Sykes, E.A.; Chan, W.C.W. Application of semiconductor and metal nanostructures in biology and medicine. Hematology 2009, 2009, 701–707. [Google Scholar] [CrossRef]
- Zadeh, D.H. Atomic excited states and the related energy levels. J. Mol. Model. 2022, 28, 282. [Google Scholar] [CrossRef]
- Caramori, G.F.; Østrøm, I.; Ortolan, A.O.; Nagurniak, G.R.; Besen, V.M.; noz Castro, A.M.; Orenha, R.P.; Parreira, R.L.T.; Galembeck, S.E. The usefulness of energy decomposition schemes to rationalize host–guest interactions. Dalton Trans. 2020, 49, 17457–17471. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A combined charge and energy decomposition scheme for bond analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Gimferrer, M.; Danés, S.; Andrada, D.M.; Salvador, P. Merging the energy decomposition analysis with the interacting quantum atoms approach. J. Chem. Theory Comput. 2023, 19, 3469–3485. [Google Scholar] [CrossRef] [PubMed]
- Mutsuji, A.; Saita, K.; Maeda, S. An energy decomposition and extrapolation scheme for evaluating electron transfer rate constants: A case study on electron self-exchange reactions of transition metal complexes. RSC Adv. 2023, 13, 32097–32103. [Google Scholar] [CrossRef] [PubMed]
- Francisco, E.; Pendás, A.M.; Blanco, M.A. A molecular energy decomposition scheme for atoms in molecules. J. Chem. Theory Comput. 2006, 1, 90–102. [Google Scholar] [CrossRef]
- Gutman, I.; Gojak, S.; Furtula, B.; Radenković, S.; Vodopivec, A. Relating total π-electron energy and resonance energy of benzenoid molecules with Kekulé- and Clar-structure-based parameters. Monatsh. Chem. 2006, 137, 1127–1138. [Google Scholar] [CrossRef]
- Gojak, S.; Gutman, I.; Radenković, S.; Vodopivec, A. Relating resonance energy with the Zhang-Zhang polynomial. J. Serb. Chem. Soc. 2007, 72, 665–671. [Google Scholar] [CrossRef]
- Kekulé, A. Sur la constitution des substances aromatiques. Bull. Soc. Chim. Fr. 1865, 2, 98–110. [Google Scholar]
- Clar, E. The aromatic sextet. In Mobile Source Emissions Including Policyclic Organic Species; Rondia, D., Cooke, M., Haroz, R.K., Eds.; Springer: Dordrecht, The Netherlands, 1983; pp. 49–58. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H. A new enumeration method for Kekulé structures of hexagonal systems with forcing edges. J. Mol. Struct. Theochem 1995, 331, 255–260. [Google Scholar] [CrossRef]
- Zhang, H.P.; Zhang, F.J. The Clar covering polynomial of hexagonal systems I. Discret. Appl. Math. 1996, 69, 147–167. [Google Scholar] [CrossRef]
- Gutman, I.; Furtula, B.; Balaban, A.T. Algorithm for simultaneous calculations of Kekulé and Clar structure counts, and Clar number of benzenoid molecules. Polycycl. Aromat. Compd. 2006, 26, 17–35. [Google Scholar] [CrossRef]
- Chou, C.P.; Witek, H.A. An algorithm and FORTRAN program for automatic computation of the Zhang-Zhang polynomial of benzenoids. MATCH Commun. Math. Comput. Chem. 2012, 68, 3–30. [Google Scholar]
- Chou, C.P.; Witek, H.A. ZZDecomposer: A graphical toolkit for analyzing the Zhang-Zhang polynomials of benzenoid structures. MATCH Commun. Math. Comput. Chem. 2014, 71, 741–764. [Google Scholar]
- Žigert Pleteršek, P. Equivalence of the generalized Zhang-Zhang polynomial and the generalized cube polynomial. MATCH Commun. Math. Comput. Chem. 2018, 80, 215–226. [Google Scholar]
- Furtula, B.; Radenković, S.; Redžepović, I.; Tratnik, N.; Žigert Pleteršek, P. The generalized Zhang–Zhang polynomial of benzenoid systems – theory and applications. Appl. Math. Comput. 2022, 418, 126822. [Google Scholar] [CrossRef]
- Gutman, I.; Borovićanin, B. Zhang-Zhang polynomial of multiple linear hexagonal chains. Z. Naturforsch. A 2006, 61, 73–77. [Google Scholar] [CrossRef]
- Chou, C.P.; Kang, J.S.; Witek, H.A. Closed-form formulas for the Zhang–Zhang polynomials of benzenoid structures: Prolate rectangles and their generalizations. Discret. Appl. Math. 2016, 198, 101–108. [Google Scholar] [CrossRef]
- Witek, H.A.; Podeszwa, R.; Langner, J. Closed–form formulas for Zhang–Zhang polynomials of hexagonal graphene flakes O(k,m,n) with k,m=1--7 and arbitrary n. MATCH Commun. Math. Comput. Chem. 2021, 83, 165–194. [Google Scholar]
- Witek, H.A. Zhang–Zhang polynomials of multiple zigzag chains revisited: A connection with the John–Sachs theorem. Molecules 2021, 26, 2524. [Google Scholar] [CrossRef]
- Gaus, M.; Cui, Q.; Elstner, M. DFTB3: Extension of the self-consistent-charge density-functional tight-binding method (SCC-DFTB). J. Chem. Theory Comput. 2011, 7, 931–948. [Google Scholar] [CrossRef]
- Gaus, M.; Goez, A.; Elstner, M. Parametrization and benchmark of DFTB3 for organic molecules. J. Chem. Theory Comput. 2013, 9, 338–354. [Google Scholar] [CrossRef]
- Hourahine, B.; Aradi, B.; Blum, V.; Bonafé, F.; Buccheri, A.; Camacho, C.; Cevallos, C.; Deshaye, M.Y.; Dumitrică, T.; Dominguez, A.; et al. DFTB+, a software package for efficient approximate density functional theory based atomistic simulations. J. Chem. Phys. 2020, 152, 124101. [Google Scholar] [CrossRef] [PubMed]
- Trefethen, L.N.; Bau, D., III. Numerical Linear Algebra; SIAM: Philadelphia, PA, USA, 1997. [Google Scholar]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in Fortran 77: The Art of Scientific Computing, 2nd ed.; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Houk, K.N.; Lee, P.S.; Nendel, M. Polyacene and cyclacene geometries and electronic structures: Bond equalization, vanishing band gaps, and triplet ground states contrast with polyacetylene. J. Org. Chem. 2001, 66, 5517–5521. [Google Scholar] [CrossRef]
- Bendikov, M.; Duong, H.M.; Starkey, K.; Houk, K.N.; Carter, E.A.; Wudl, F. Oligoacenes: Theoretical prediction of open-shell singlet diradical ground states. J. Am. Chem. Soc. 2004, 126, 7416–7417. [Google Scholar] [CrossRef]
- Jiang, D.; Dai, S. Electronic ground state of higher acenes. J. Phys. Chem. A 2008, 112, 332–335. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, D.; Liu, C.; Jiang, Y. Open-shell ground state of polyacenes: A valence bond study. J. Phys. Chem. A 2009, 113, 7909–7914. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Small, D.W.; Epifanovsky, E.; Head-Gordon, M. Coupled-cluster valence-bond singles and doubles for strongly correlated systems: Block-tensor based implementation and application to oligoacenes. J. Chem. Theory Comput. 2017, 13, 602–615. [Google Scholar] [CrossRef]
- Das, A.; Müller, T.; Plasser, F.; Lischka, H. Polyradical character of triangular non-Kekulé structures, zethrenes, p-quinodimethane-linked bisphenalenyl, and the Clar goblet in comparison: An extended multireference study. J. Phys. Chem. A 2016, 120, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.N.; Chai, J.D. Role of Kekulé and non-Kekulé structures in the radical character of alternant polycyclic aromatic hydrocarbons: A TAO-DFT study. Sci. Rep. 2016, 6, 30562. [Google Scholar] [CrossRef] [PubMed]
- Plasser, F.; Pašalić, H.; Gerzabek, M.H.; Libisch, F.; Reiter, R.; Burgdörfer, J.; Müller, T.; Shepard, R.; Lischka, H. The multiradical character of one- and two-dimensional graphene nanoribbons. Angew. Chem. Int. Ed. 2013, 52, 2581–2584. [Google Scholar] [CrossRef] [PubMed]
- Urgel, J.I.; Mishra, S.; Hayashi, H.; Wilhelm, J.; Pignedoli, C.A.; Giovannantonio, M.D.; Widmer, R.; Yamashita, M.; Hieda, N.; Ruffieux, P.; et al. On-surface light-induced generation of higher acenes and elucidation of their open-shell character. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Trinquier, G.; David, G.; Malrieu, J.P. Qualitative views on the polyradical character of long acenes. J. Phys. Chem. A 2018, 122, 6926–6933. [Google Scholar] [CrossRef]
- Minkin, V.I.; Starikov, A.G.; Starikova, A.A. Acene-linked zethrenes and bisphenalenyls: A DFT search for organic tetraradicals. J. Phys. Chem. A 2021, 125, 6562–6570. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, Y.; Chen, G. First–principles study of potassium adsorption and diffusion on graphene. Mol. Phys. 2019, 118, e1581291. [Google Scholar] [CrossRef]
- Koh, Y.; Manzhos, S. Curvature drastically changes diffusion properties of Li and Na on graphene. MRS Commun. 2013, 3, 171–175. [Google Scholar] [CrossRef]
- Flores, M.Z.S.; Autreto, P.A.S.; Legoas, S.B.; Galvao, D.S. Graphene to graphane: A theoretical study. Nanotechnology 2009, 20, 465704. [Google Scholar] [CrossRef] [PubMed]
- Malyi, O.I.; Sopiha, K.; Kulish, V.V.; Tan, T.L.; Manzhos, S.; Persson, C. A computational study of Na behavior on graphene. Appl. Surf. Sci. 2015, 333, 235–243. [Google Scholar] [CrossRef]
- Yen, T.F. Resonance topology of polynuclear aromatic hydrocarbons. Theor. Chim. Acta 1971, 20, 399–404. [Google Scholar] [CrossRef]
- Gutman, I. Topological properties of benzenoid systems. XXVIII. Number of Kekulé structures of some benzenoid hydrocarbons. MATCH Commun. Math. Comput. Chem. 1985, 17, 3–10. [Google Scholar]
- He, B.H.; Langner, J.; Witek, H.A. Hexagonal flakes as fused parallelograms: A determinantal formula for Zhang-Zhang polynomials of the O(2,m,n) benzenoids. J. Chin. Chem. Soc. 2021, 68, 1231–1242. [Google Scholar] [CrossRef]
- He, B.H.; Langner, J.; Podeszwa, R.; Witek, H.A. Can the John–Sachs theorem be extended to Clar covers? MATCH Commun. Math. Comput. Chem. 2021, 83, 141–163. [Google Scholar]
- Langner, J.; Witek, H.A. Interface theory of benzenoids. MATCH Commun. Math. Comput. Chem. 2020, 84, 143–176. [Google Scholar]
- Langner, J.; Witek, H.A. Interface theory of benzenoids: Basic applications. MATCH Commun. Math. Comput. Chem. 2020, 84, 177–215. [Google Scholar]
- Langner, J.; Witek, H.A. ZZ polynomials of regular m-tier benzenoid strips as extended strict order polynomials of associated posets. Part 1. Proof of equivalence. MATCH Commun. Math. Comput. Chem. 2022, 87, 585–620. [Google Scholar] [CrossRef]









| m | n | (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|
| 25 | 25 | |||||||
| 30 | 30 | |||||||
| 35 | 35 | |||||||
| 26 | 30 | |||||||
| 30 | 26 | |||||||
| 27 | 35 | |||||||
| 35 | 27 | |||||||
| 31 | 35 | |||||||
| 35 | 31 | |||||||
| MAE | ||||||||
| Set | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | |||||||||
| 1 | … | ||||||||
| 1 | … | ||||||||
| 7 | … | ||||||||
| 10 | … | ||||||||
| 10 | … | ||||||||
| 10 | … | ||||||||
| 10 | … | ||||||||
| 10 | … | ||||||||
| 10 | |||||||||
| 10 | … | ||||||||
| 10 | |||||||||
| 10 | |||||||||
| () | () | () | () | () | () | () | () | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendra; Witek, H.A. Energy Decomposition Scheme for Rectangular Graphene Flakes. Nanomaterials 2024, 14, 181. https://doi.org/10.3390/nano14020181
Hendra, Witek HA. Energy Decomposition Scheme for Rectangular Graphene Flakes. Nanomaterials. 2024; 14(2):181. https://doi.org/10.3390/nano14020181
Chicago/Turabian StyleHendra, and Henryk A. Witek. 2024. "Energy Decomposition Scheme for Rectangular Graphene Flakes" Nanomaterials 14, no. 2: 181. https://doi.org/10.3390/nano14020181