Sorption and Desorption of Vapor of n-Pentane by Porphyrin Aluminum Metal–Organic Framework: Mechanism of Bonding, Kinetics and Stoichiometry by Complementary In-Situ Time-Dependent and Ex-Situ Methods
Abstract
:1. Introduction
2. Materials and methods
2.1. Chemicals
2.2. Synthesis of asisAl-MOF-TCPPH2 and Its Thermal Activation to actAl-MOF-TCPPH2
2.3. Instrumental Characterization of Specimens
2.4. Gas Flow Chamber Attachment on FTIR Spectrometer
2.5. Setup for Preparation of Flow of Dried Air and Dried Air Saturated with n-Pentane
2.6. Dynamic Sorption of n-Pentane Vapor by Compound 2 using In-Situ Time-Dependent ATR-FTIR Spectroscopy in Controlled Dry Atmosphere
2.7. Dynamic Sorption of n-Pentane Vapor by Compound 2 Studied by In-Situ Time-Dependent Gravimetry
2.8. Static (in Saturated Vapor) Sorption of n-Pentane by Compound 2
2.9. Dynamic Desorption of n-Pentane Vapor by Compound 3 Studied by In-Situ Time-Dependent Gravimetry
3. Results and Discussion
3.1. Setting up the Flow Chamber for In-Situ Time-Dependent ATR-FTIR Spectroscopic Study of Sorption of n-Pentane Vapor by Compound 2 at Controlled Low Humidity
3.2. Dynamics of Sorption of n-Pentane Vapor by Compound 2 Studied by In-Situ Time-Dependent ATR-FTIR Spectroscopy in Controlled Dry Atmosphere
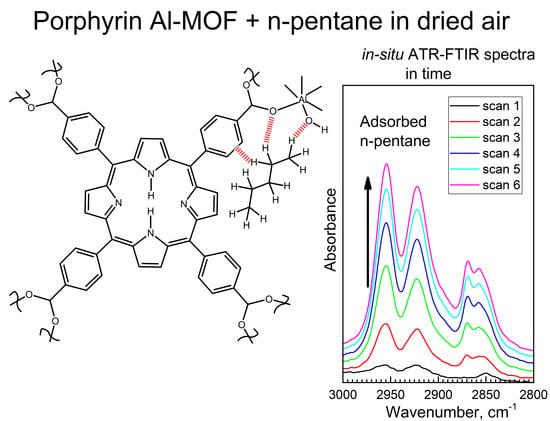
3.3. The In-Situ Time-Dependent ATR-FTIR Spectra to Find Specific Functional Groups in Compound 2 Interacting with n-Pentane
3.4. Kinetics of Sorption of n-Pentane Vapor by Compound 2 Using In-Situ Time-Dependent Gravimetric Analysis
3.5. The Ex-Situ (Static) Sorption of n-Pentane Vapor in Dried Air by Compound 2
3.6. Kinetics of Desorption of n-Pentane by Compound 3 by In-Situ Time-Dependent Gravimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samokhvalov, A. Adsorption on Mesoporous Metal-Organic Frameworks in Solution for Clean Energy, Environment and Healthcare; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Li, X.; Liu, J.; Zhou, K.; Ullah, S.; Wang, H.; Zou, J.; Thonhauser, T.; Li, J. Tuning Metal–Organic Framework (MOF) Topology by Regulating Ligand and Secondary Building Unit (SBU) Geometry: Structures Built on 8-Connected M6 (M = Zr, Y) Clusters and a Flexible Tetracarboxylate for Propane-Selective Propane/Propylene Separation. J. Am. Chem. Soc. 2022, 144, 21702–21709. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Yang, J.; Yang, R.T. Investigation on Hydrogenation of Metal–Organic Frameworks HKUST-1, MIL-53, and ZIF-8 by Hydrogen Spillover. J. Phys. Chem. C 2013, 117, 7565–7576. [Google Scholar] [CrossRef]
- Saha, D.; Deng, S. Ammonia adsorption and its effects on framework stability of MOF-5 and MOF-177. J. Colloid Interface Sci. 2010, 348, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Coudert, F.-X.; Ortiz, A.U.; Haigis, V.; Bousquet, D.; Fuchs, A.H.; Ballandras, A.; Weber, G.; Bezverkhyy, I.; Geoffroy, N.; Bellat, J.-P.; et al. Water Adsorption in Flexible Gallium-Based MIL-53 Metal–Organic Framework. J. Phys. Chem. C 2014, 118, 5397–5405. [Google Scholar] [CrossRef]
- Ou, R.; Zhang, H.; Truong, V.X.; Zhang, L.; Hegab, H.M.; Han, L.; Hou, J.; Zhang, X.; Deletic, A.; Jiang, L.; et al. A sunlight-responsive metal–organic framework system for sustainable water desalination. Nat. Sustain. 2020, 3, 1052–1058. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Hanikel, N.; Lyle, S.J.; Zhu, C.; Proserpio, D.M.; Yaghi, O.M. A Porous Covalent Organic Framework with Voided Square Grid Topology for Atmospheric Water Harvesting. J. Am. Chem. Soc. 2020, 142, 2218–2221. [Google Scholar] [CrossRef]
- Samokhvalov, A. Aluminum metal–organic frameworks for sorption in solution: A review. Coord. Chem. Rev. 2018, 374, 236–253. [Google Scholar] [CrossRef]
- Henry, B.; Samokhvalov, A. Hygroscopic metal-organic framework MIL-160(Al): In-situ time-dependent ATR-FTIR and gravimetric study of mechanism and kinetics of water vapor sorption. Spectrochim. Acta A 2022, 267, 120550. [Google Scholar] [CrossRef]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Ferey, G. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Dong, L.Z.; Xu, Y.; Han, W.; Fang, M.; Liu, H.K.; Wu, Y.; Lan, Y.Q. Syntheses of Exceptionally Stable Aluminum(III) Metal–Organic Frameworks: How to Grow High Quality, Large, Single Crystals. Chem. Eur. J. 2017, 23, 15518–15528. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Sun, F.; Xie, J.; Pan, Q. New challenge of microporous metal-organic frameworks for adsorption of hydrogen fluoride gas. Mater. Lett. 2017, 197, 175–179. [Google Scholar] [CrossRef]
- Biesaga, M.; Pyrzyńska, K.; Trojanowicz, M. Porphyrins in analytical chemistry. A review. Talanta 2000, 51, 209–224. [Google Scholar] [CrossRef]
- Nakashima, S.; Negishi, R.; Tada, H. Visible-light-induced water oxidation by a hybrid photocatalyst consisting of bismuth vanadate and copper(II) meso-tetra(4-carboxyphenyl)porphyrin. Chem. Commun. 2016, 52, 3665–3668. [Google Scholar] [CrossRef] [PubMed]
- Kosal, M.E. Terrorism Targeting Industrial Chemical Facilities: Strategic Motivations and the Implications for U.S. Security. Stud. Confl. Terror. 2006, 29, 719–751. [Google Scholar] [CrossRef]
- Wu, H.; Gong, Q.; Olson, D.H.; Li, J. Commensurate Adsorption of Hydrocarbons and Alcohols in Microporous Metal Organic Frameworks. Chem. Rev. 2012, 112, 836–868. [Google Scholar] [CrossRef]
- Gismondi, P.; Kuzmin, A.; Unsworth, C.; Rangan, S.; Khalid, S.; Saha, D. Understanding the Adsorption of Rare-Earth Elements in Oligo-Grafted Mesoporous Carbon. Langmuir 2022, 38, 203–210. [Google Scholar] [CrossRef]
- Dai, J.; McKee, M.L.; Samokhvalov, A. Adsorption of naphthalene and indole on F300 MOF in liquid phase by the complementary spectroscopic, kinetic and DFT studies. J. Porous Mater. 2014, 21, 709–727. [Google Scholar] [CrossRef]
- Salazar, J.M.; Weber, G.; Simon, J.M.; Bezverkhyy, I.; Bellat, J.P. Characterization of adsorbed water in MIL-53(Al) by FTIR spectroscopy and ab-initio calculations. J. Chem. Phys. 2015, 142, 124702. [Google Scholar] [CrossRef]
- Tumuluri, U.; Isenberg, M.; Tan, C.-S.; Chuang, S.S.C. In Situ Infrared Study of the Effect of Amine Density on the Nature of Adsorbed CO2 on Amine-Functionalized Solid Sorbents. Langmuir 2014, 30, 7405–7413. [Google Scholar] [CrossRef]
- Wilfong, W.C.; Srikanth, C.S.; Chuang, S.S.C. In Situ ATR and DRIFTS Studies of the Nature of Adsorbed CO2 on Tetraethylenepentamine Films. ACS Appl. Mater. Interfaces 2014, 6, 13617–13626. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, I.P.; Keyworth, C.W.; Brown, N.J.; Shaffer, M.S.P.; Williams, C.K.; Hellgardt, K.; Kelsall, G.H.; Kazarian, S.G. An Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopic Study of Gas Adsorption on Colloidal Stearate-Capped ZnO Catalyst Substrate. Appl. Spectrosc. 2014, 68, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Han, X.; Chu, J.; Xiong, J.; He, X.; Wang, H.-L.; Xu, P. In Situ Surface-Enhanced Raman Spectroscopy Study of Plasmon-Driven Catalytic Reactions of 4-Nitrothiophenol under a Controlled Atmosphere. ChemCatChem 2015, 7, 1004–1010. [Google Scholar] [CrossRef]
- Wei, G. Electrostatic Control and Air Ionization in Cleanrooms for Semiconductor and TFT Production. J. IEST 2009, 52, 87–97. [Google Scholar] [CrossRef]
- Shelaev, A.; Sgibnev, Y.; Efremova, S.; Tananaev, P.; Baryshev, A. Micron-scale crystallization of Bi:YIG by laser rapid thermal annealing at controlled atmosphere. Opt. Laser Technol. 2022, 155, 108411. [Google Scholar] [CrossRef]
- Thornblom, J.; Roihjert, K.-J. Utilizing plasma technology for chemical reactions in controlled atmosphere. Pure Appl. Chem. 1992, 64, 671–676. [Google Scholar] [CrossRef]
- Gong, Z.; Pan, Y.-L.; Videen, G.; Wang, C. Chemical reactions of single optically trapped bioaerosols in a controlled environment. Aerosol Sci. Technol. 2019, 53, 853–859. [Google Scholar] [CrossRef]
- Ribeiro, S.R.; Klein, B.; Santos, I.D.d.; Thewes, F.R.; Brackmann, A.; Both, V.; Wagner, R. Effects of controlled atmosphere and storage temperature on the quality of shelled ‘Barton’ pecan nuts during long-term storage. Food Res. Int. 2022, 158, 111498. [Google Scholar] [CrossRef]
- Guerra, D.; Ricciardi, L.; Laborde, J.-C.; Domenech, S. Predicting Gaseous Pollutant Dispersion Around a Workplace. J. Occup. Environ. Hyg. 2007, 4, 619–633. [Google Scholar] [CrossRef]
- Mi, R.; Li, D.; Hu, Z.; Yang, R.T. Morphology Effects of CeO2 Nanomaterials on the Catalytic Combustion of Toluene: A Combined Kinetics and Diffuse Reflectance Infrared Fourier Transform Spectroscopy Study. ACS Catal. 2021, 11, 7876–7889. [Google Scholar] [CrossRef]
- Banga-Bothy, G.-A.; Samokhvalov, A. Porphyrin aluminum MOF with ultra-high water sorption capacity: In-situ time-dependent ATR-FTIR spectroscopy and gravimetry to study mechanism of water bonding and desorption. Vib. Spectrosc. 2022, 119, 103356. [Google Scholar] [CrossRef]
- Samokhvalov, A.; McCombs, S. In Situ Time-Dependent Attenuated Total Reflection Fourier Transform Infrared (ATR FT-IR) Spectroscopy of a Powdered Specimen in a Controlled Atmosphere: Monitoring Sorption and Desorption of Water Vapor. Appl. Spectrosc. 2023, 77, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Padial, N.M.; Quartapelle Procopio, E.; Montoro, C.; López, E.; Oltra, J.E.; Colombo, V.; Maspero, A.; Masciocchi, N.; Galli, S.; Senkovska, I.; et al. Highly Hydrophobic Isoreticular Porous Metal–Organic Frameworks for the Capture of Harmful Volatile Organic Compounds. Angew. Chem. Int. Ed. 2013, 52, 8290–8294. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Sunanda, K.; Rajasekhar, B.N. Spectroscopy of structural isomers of pentanes: An experimental and theoretical study. J. Mol. Struct. 2021, 1245, 131126. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Menapace, J.A.; Bernstein, E.R. Van der Waals modes of solute/solvent clusters: Benzene-methane, -deuteriomethane, and -carbon tetrafluoride. J. Phys. Chem. 1987, 91, 2843–2848. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, P.; Liu, J.; Shen, F.; Zhang, Y.; Chai, K.; Ying, Y.; Kang, C.; Zhang, Z.; Ji, H. Enhancing CH4/N2 separation performance within aluminum-based Metal-Organic Frameworks: Influence of the pore structure and linker polarity. Sep. Purif. Technol. 2022, 286, 120446. [Google Scholar] [CrossRef]
- Wischert, R.; Copéret, C.; Delbecq, F.; Sautet, P. Optimal Water Coverage on Alumina: A Key to Generate Lewis Acid–Base Pairs that are Reactive Towards the C-H Bond Activation of Methane. Angew. Chem. Int. Ed. 2011, 50, 3202–3205. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A Water-Stable Porphyrin-Based Metal–Organic Framework Active for Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banga-Bothy, G.-A.; Samokhvalov, A. Sorption and Desorption of Vapor of n-Pentane by Porphyrin Aluminum Metal–Organic Framework: Mechanism of Bonding, Kinetics and Stoichiometry by Complementary In-Situ Time-Dependent and Ex-Situ Methods. Nanomaterials 2023, 13, 1529. https://doi.org/10.3390/nano13091529
Banga-Bothy G-A, Samokhvalov A. Sorption and Desorption of Vapor of n-Pentane by Porphyrin Aluminum Metal–Organic Framework: Mechanism of Bonding, Kinetics and Stoichiometry by Complementary In-Situ Time-Dependent and Ex-Situ Methods. Nanomaterials. 2023; 13(9):1529. https://doi.org/10.3390/nano13091529
Chicago/Turabian StyleBanga-Bothy, Georgia-Annicette, and Alexander Samokhvalov. 2023. "Sorption and Desorption of Vapor of n-Pentane by Porphyrin Aluminum Metal–Organic Framework: Mechanism of Bonding, Kinetics and Stoichiometry by Complementary In-Situ Time-Dependent and Ex-Situ Methods" Nanomaterials 13, no. 9: 1529. https://doi.org/10.3390/nano13091529