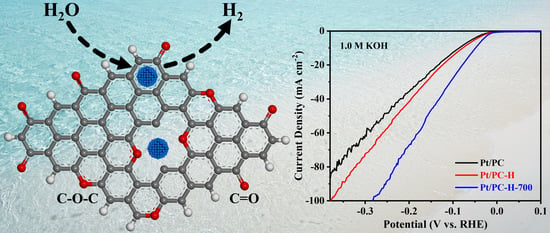
The Enhancing Effect of Stable Oxygen Functional Groups on Porous-Carbon-Supported Pt Catalysts for Alkaline Hydrogen Evolution
Abstract
:Highlights
- The HCl treatment of porous carbon can generate abundant hydroxyl and carboxyl groups, while the further heat treatment can transform into thermally stable carbonyl and ether groups.
- Carbonyl and ether groups within porous carbon supports is beneficial to the improvement of HER performance of catalysts.
- The surface properties of porous carbon supports can be well tuned via a HCl treatment followed by an appropriate heat treatment.
- The feasible improvement of HER performance by regulating surface oxygen functional groups of porous carbon supports.
Abstract
1. Introduction
2. Experimental
2.1. HCl Treatment of PC
2.2. Heat Treatment of PC-H
2.3. Preparation of Pt/C Catalysts
2.4. Structural Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ola, O.; Zhao, J.; Yang, Z.; Tiwari, S.K.; Wang, N.; Zhu, Y. Recent progress in graphene-based electrocatalysts for hydrogen evolution reaction. Nanomaterials 2022, 12, 1806. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.R.; Xiao, Y.Y.; Yin, Y.C.; Huang, W.M.; Huang, Z.C.; Zheng, Y.Z.; Mu, F.Y.; Huang, R.; Shi, G.Y.; et al. Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Gerber, I.C.; Serp, P. A theory/experience description of support effects in carbon-supported catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zheng, H.; Lai, W.; Yuan, P.; Li, S.; Li, D.; Chen, Y. Defect engineering of carbons for energy conversion and storage applications. Energy Environ. Mater. 2023, 6, 1–22. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Zhang, M.; Gou, W.; Zhang, S.; Chen, Z.; Qu, Y.; Ma, Y. A fundamental viewpoint on the hydrogen spillover phenomenon of electrocatalytic hydrogen evolution. Nat. Commun. 2021, 12, 3502. [Google Scholar] [CrossRef]
- Fang, L.; Yang, B.; Cai, J.; Feng, Y.; Li, X.; Li, Y. Electrochemical modification and structural characterization of porous PtNi/C catalyst. J. Alloy. Compd. 2021, 879, 160454. [Google Scholar] [CrossRef]
- Kuang, P.; Wang, Y.; Zhu, B.; Xia, F.; Tung, C.W.; Wu, J.; Chen, H.M.; Yu, J. Pt single atoms supported on N-doped mesoporous hollow carbon spheres with enhanced electrocatalytic H2-evolution activity. Adv. Mater. 2021, 33, e2008599. [Google Scholar] [CrossRef] [PubMed]
- Holade, Y.; Morais, C.; Servat, K.; Napporn, T.W.; Kokoh, K.B. Enhancing the available specific surface area of carbon supports to boost the electroactivity of nanostructured Pt catalysts. Phys. Chem. Chem. Phys. 2014, 16, 25609–25620. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Hu, C.; Wang, G.; Zou, Z.; Yang, H.; Dai, L. Carbon-defect-driven electroless deposition of Pt atomic clusters for highly efficient hydrogen evolution. J. Am. Chem. Soc. 2020, 142, 5594–5601. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, H.; Yuan, P.; Jia, Y.; Zhuang, L.; Zhang, H.; Yan, X.; Liu, G.; Zhao, Y.; Liu, J.; et al. Single carbon vacancy traps atomic platinum for hydrogen evolution catalysis. J. Am. Chem. Soc. 2022, 144, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, S.; Hu, T.; Chen, Y.; Li, F. Renewable biomass-derived carbons for electrochemical capacitor applications. SusMat 2021, 1, 211–240. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Yin, H.; Shi, W.; Waterhouse, G.I.N.; Li, H.; Ai, S. Yolk-shell Fe3O4 nanoparticles loaded on persimmon-derived porous carbon for supercapacitor assembly and As (V) removal. J. Alloy. Compd. 2019, 810, 151887. [Google Scholar] [CrossRef]
- Kim, J.H.; Cheon, J.Y.; Shin, T.J.; Park, J.Y.; Joo, S.H. Effect of surface oxygen functionalization of carbon support on the activity and durability of Pt/C catalysts for the oxygen reduction reaction. Carbon 2016, 101, 449–457. [Google Scholar] [CrossRef]
- Shrestha, S.; Liu, Y.; Mustain, W.E. Electrocatalytic activity and stability of Pt clusters on state-of-the-art supports: A review. Catal. Rev. 2011, 53, 256–336. [Google Scholar] [CrossRef]
- Schmies, H.; Hornberger, E.; Anke, B.; Jurzinsky, T.; Nong, H.N.; Dionigi, F.; Kühl, S.; Drnec, J.; Lerch, M.; Cremers, C.; et al. Impact of carbon support functionalization on the electrochemical stability of Pt fuel cell catalysts. Chem. Mater. 2018, 30, 7287–7295. [Google Scholar] [CrossRef]
- Ying, J.; Jiang, G.; Paul Cano, Z.; Han, L.; Yang, X.Y.; Chen, Z. Nitrogen-doped hollow porous carbon polyhedrons embedded with highly dispersed Pt nanoparticles as a highly efficient and stable hydrogen evolution electrocatalyst. Nano Energy 2017, 40, 88–94. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.; Tang, L.; Liu, C.; Gao, X.; Sun, M.; Zheng, J.; Ling, M.; Liang, C.; Lin, Z. Platinum single-atom and cluster anchored on functionalized MWCNTs with ultrahigh mass efficiency for electrocatalytic hydrogen evolution. Nano Energy 2019, 63, 103849. [Google Scholar] [CrossRef]
- Zeng, X.; Shui, J.; Liu, X.; Liu, Q.; Li, Y.; Shang, J.; Zheng, L.; Yu, R. Single-atom to single-atom grafting of Pt1 onto Fe-N4 center: Pt1@Fe-N-C multifunctional electrocatalyst with significantly enhanced properties. Adv. Energy Mater. 2018, 8, 1701345. [Google Scholar] [CrossRef]
- Chen, W.; Xin, Q.; Sun, G.; Wang, Q.; Mao, Q.; Su, H. The effect of carbon support treatment on the stability of Pt/C electrocatalysts. J. Power Sources 2008, 180, 199–204. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.J. Effects of chemical treatment of carbon supports on electrochemical behaviors for platinum catalysts of fuel cells. J. Power Sources 2006, 159, 42–45. [Google Scholar] [CrossRef]
- Calvillo, L.; Gangeri, M.; Perathoner, S.; Centi, G.; Moliner, R.; Lázaro, M.J. Effect of the support properties on the preparation and performance of platinum catalysts supported on carbon nanofibers. J. Power Sources 2009, 192, 144–150. [Google Scholar] [CrossRef]
- Yin, S.; Shen, P.K.; Song, S.; Jiang, S.P. Functionalization of carbon nanotubes by an effective intermittent microwave heating-assisted HF/H2O2 treatment for electrocatalyst support of fuel cells. Electrochim. Acta 2009, 54, 6954–6958. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, K.; Si, M.; Wang, H.; Chai, L.; Shi, Y. Three-dimensional carbon nanosheets derived from micro-morphologically regulated biomass for ultrahigh-performance supercapacitors. Carbon 2019, 153, 707–716. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, N.; Chong, S.; Li, D.; Chen, Y.; Sun, C. Three-dimensional porous graphene-like sheets synthesized from biocarbon via low-temperature graphitization for a supercapacitor. Green Chem. 2018, 20, 694–700. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, S.; Wu, P.; Wang, H.; Huang, Y.; Zhang, Y.; Sun, D.; Tang, Y.; Wang, H. Engineering surface oxygenated functionalities on commercial hard carbon toward superior sodium storage. Chem. Eng. J. 2022, 441, 135899. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, W.; Song, M.; Zhang, J.; He, F.; Wang, J.; Xiong, M.; Zhang, J.; Wang, D. Pyranoid-O-dominated graphene-like nanocarbon for two-electron oxygen reduction reaction. Appl. Catal. B Environ. 2022, 307, 121173. [Google Scholar] [CrossRef]
- Peng, H.; Yao, B.; Wei, X.; Liu, T.; Kou, T.; Xiao, P.; Zhang, Y.; Li, Y. Pore and heteroatom engineered carbon foams for supercapacitors. Adv. Energy Mater. 2019, 9, 1803665. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, T.; Mo, Y.; Li, D.; Zheng, C.; Chen, Y. Three-dimensional stacked graphite sheets with exposed edge-defects as Pt-based catalyst support. Electrochim. Acta 2022, 404, 139602. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, S.; Zhou, W.; Wang, G.; Jiang, L.; Li, W.; Song, S.; Liu, J.; Sun, G.; Xin, Q. Novel synthesis of highly active Pt/C cathode electrocatalyst for direct methanol fuel cell. Chem. Commun. 2003, 394–395. [Google Scholar] [CrossRef]
- Thommes, M. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2016, 38, 25. [Google Scholar] [CrossRef]
- Qiu, D.; Li, M.; Kang, C.; Wei, J.; Wang, F.; Yang, R. Cucurbit [6]uril-derived sub-4 nm pores-dominated hierarchical porous carbon for supercapacitors: Operating voltage expansion and pore size matching. Small 2020, 16, e2002718. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.Y.; Zhao, Z.B.; Song, Y.Z.; Sun, L.L.; Fan, Z.J.; Yang, Y.Z.; Liu, X.G.; Wang, X.Z.; Qiu, J.S. 3D carbon frameworks for ultrafast charge/discharge rate supercapacitors with high energy-power density. Nano-Micro Lett. 2021, 13, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, J.; Song, S.; Zhou, Y.; Li, L. Electrolyte-dependent supercapacitor performance on nitrogen-doped porous bio-carbon from gelatin. Nanomaterials 2020, 10, 353. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Ganesan, K.; Ghosh, S.; Gopala Krishna, N.; Ilango, S.; Kamruddin, M.; Tyagi, A.K. A comparative study on defect estimation using XPS and Raman spectroscopy in few layer nanographitic structures. Phys. Chem. Chem. Phys. 2016, 18, 22160–22167. [Google Scholar] [CrossRef]
- Cancado, L.G.; Jorio, A.; Ferreira, E.H.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Acik, M.; Mattevi, C.; Gong, C.; Lee, G.; Cho, K.; Chhowalla, M.; Chabal, Y.J. The role of intercalated water in multilayered graphene oxide. ACS Nano 2010, 4, 5861–5868. [Google Scholar] [CrossRef]
- Zhu, F.; Li, J.; Zhu, M.; Kang, L. Effect of oxygen-containing group on the catalytic performance of Zn/C catalyst for acetylene acetoxylation. Nanomaterials 2021, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Abbas, M.A.; Bang, J.H. Exploring the capacitive behavior of carbon functionalized with cyclic ethers: A rational strategy to exploit oxygen functional groups for enhanced capacitive performance. ACS Appl. Mater. Interfaces 2019, 11, 14126–14135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Jiang, Y.H.; Wang, P.Z.; Mo, Y.; Lai, W.D.; Li, Z.J.; Yu, R.J.; Du, Y.T.; Zhang, X.R.; Chen, Y. Effect of the oxygen functional groups of activated carbon on its electrochemical performance for supercapacitors. New Carbon Mater. 2020, 35, 232–243. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, X.; Wang, H.; Xie, L.; Cheng, J.; Kong, Q.; Sun, G.; Chen, C.M. Structure evolution of oxygen removal from porous carbon for optimizing supercapacitor performance. J. Energy Chem. 2020, 51, 396–404. [Google Scholar] [CrossRef]
- Han, G.F.; Li, F.; Zou, W.; Karamad, M.; Jeon, J.P.; Kim, S.W.; Kim, S.J.; Bu, Y.; Fu, Z.; Lu, Y.; et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 2020, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Ren, Y.; Wang, Y.; Mo, L.; Li, Z.; Zhang, H.; Hu, L.; Cao, G. Addition of dioxane in electrolyte promotes (002)-textured zinc growth and suppressed side reactions in zinc-ion batteries. ACS Nano 2023, 17, 3765–3775. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, G.; Siahrostami, S.; Chen, Z.; Liu, K.; Xie, J.; Liao, L.; Wu, T.; Lin, D.; Liu, Y.; et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 2018, 1, 156–162. [Google Scholar] [CrossRef]
- Fan, Y.F.; Yi, Z.L.; Song, G.; Wang, Z.F.; Chen, C.J.; Xie, L.J.; Sun, G.H.; Su, F.Y.; Chen, C.M. Self-standing graphitized hybrid Nanocarbon electrodes towards high-frequency supercapacitors. Carbon 2021, 185, 630–640. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Wang, Y.; Dou, S.; Yan, D.; Liu, D.; Xia, Z.; Wang, S. In situ exfoliated, edge-rich, oxygen-functionalized graphene from carbon fibers for oxygen electrocatalysis. Adv. Mater. 2017, 29, 1606207. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, S.; Du, X.; Hong, S.; Zhao, S.; Chen, Y.; Chen, X.; Song, H. Boosting the electrical double-layer capacitance of graphene by self-doped defects through ball-milling. Adv. Funct. Mater. 2019, 29, 1901127. [Google Scholar] [CrossRef]
- Wu, X.S.; Dong, X.L.; Wang, B.Y.; Xia, J.L.; Li, W.C. Revealing the sodium storage behavior of biomass-derived hard carbon by using pure lignin and cellulose as model precursors. Renew. Energ. 2022, 189, 630–638. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Liu, X.; Li, L. Biomass derived interconnected hierarchical micro-meso-macro- porous carbon with ultrahigh capacitance for supercapacitors. Carbon 2019, 147, 540–549. [Google Scholar] [CrossRef]
- Liu, M.; Niu, J.; Zhang, Z.; Dou, M.; Li, Z.; Wang, F. Porous carbons with tailored heteroatom doping and well-defined porosity as high-performance electrodes for robust Na-ion capacitors. J. Power Sources 2019, 414, 68–75. [Google Scholar] [CrossRef]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Sandbeck, D.J.S.; Secher, N.M.; Speck, F.D.; Sørensen, J.E.; Kibsgaard, J.; Chorkendorff, I.; Cherevko, S. Particle size effect on platinum dissolution: Considerations for accelerated stability testing of fuel cell catalysts. ACS Catal. 2020, 10, 6281–6290. [Google Scholar] [CrossRef]
- Zhong, H.; Luo, X.; Chen, H.; Huang, S.; Chen, Y.; Li, D. Pt3Ni alloy catalyst coupled with three-dimensional nitrogen-doped graphene for enhancing the alkaline hydrogen evolution. Electrochim. Acta 2022, 429, 141030. [Google Scholar] [CrossRef]
- Luo, X.Y.; Chen, Y.; Mo, Y. A review of charge storage in porous carbon-based supercapacitors. New Carbon Mater. 2021, 36, 49–68. [Google Scholar] [CrossRef]
- Santos, J.L.; Centeno, M.A.; Odriozola, J.A. Reductant atmospheres during slow pyrolysis of cellulose: First approach to obtaining efficient char-based catalysts in one pot. J. Anal. Appl. Pyrol. 2020, 148, 104821. [Google Scholar] [CrossRef]
- Santos, J.L.; Sanz-Moral, L.M.; Aho, A.; Ivanova, S.; Murzin, D.Y.; Centeno, M.A. Structure effect of modified biochar in Ru/C catalysts for sugar mixture hydrogenation. Biomass Bioenergy 2022, 163, 106504. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, Y.; Kim, B.S. Multifunctional role of reduced graphene oxide binder for high performance supercapacitor with commercial-level mass loading. J. Power Sources 2020, 454, 227917. [Google Scholar] [CrossRef]
- Deng, L.; Goh, K.; Yu, F.D.; Xia, Y.; Jiang, Y.S.; Ke, W.; Han, Y.; Que, L.F.; Zhou, J.; Wang, Z.B. Self-optimizing weak solvation effects achieving faster low-temperature charge transfer kinetics for high-voltage Na3V2(PO4)2F3 cathode. Energy Storage Mater. 2022, 44, 82–92. [Google Scholar] [CrossRef]







| Supports | a SBET (m2·g−1) | b SQSDFT (m2·g−1) | c Smicro (m2·g−1) | d Sexternal (m2·g−1) | e Vtotal (cm3·g−1) | f VQSDFT (cm3·g−1) | g Vmicro (cm3·g−1) | h Vexternal (cm3·g−1) |
|---|---|---|---|---|---|---|---|---|
| PC | 1751 | 1605 | 1501 | 104 | 0.94 | 0.90 | 0.73 | 0.17 |
| PC-H | 1796 | 1636 | 1517 | 119 | 0.99 | 0.95 | 0.75 | 0.20 |
| PC-H-500 | 1857 | 1663 | 1570 | 93 | 0.97 | 0.92 | 0.77 | 0.15 |
| PC-H-600 | 1809 | 1640 | 1523 | 117 | 0.99 | 0.95 | 0.76 | 0.19 |
| PC-H-700 | 1888 | 1650 | 1538 | 113 | 1.00 | 0.96 | 0.78 | 0.18 |
| PC-H-800 | 1845 | 1647 | 1546 | 101 | 0.96 | 0.92 | 0.76 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Yuan, P.; Luo, J.; Xiao, H.; Li, J.; Zheng, H.; Du, B.; Li, D.; Chen, Y. The Enhancing Effect of Stable Oxygen Functional Groups on Porous-Carbon-Supported Pt Catalysts for Alkaline Hydrogen Evolution. Nanomaterials 2023, 13, 1415. https://doi.org/10.3390/nano13081415
Luo X, Yuan P, Luo J, Xiao H, Li J, Zheng H, Du B, Li D, Chen Y. The Enhancing Effect of Stable Oxygen Functional Groups on Porous-Carbon-Supported Pt Catalysts for Alkaline Hydrogen Evolution. Nanomaterials. 2023; 13(8):1415. https://doi.org/10.3390/nano13081415
Chicago/Turabian StyleLuo, Xianyou, Ping Yuan, Junhui Luo, Haoming Xiao, Junyi Li, Heng Zheng, Baodong Du, De Li, and Yong Chen. 2023. "The Enhancing Effect of Stable Oxygen Functional Groups on Porous-Carbon-Supported Pt Catalysts for Alkaline Hydrogen Evolution" Nanomaterials 13, no. 8: 1415. https://doi.org/10.3390/nano13081415