Nanomaterial with Core–Shell Structure Composed of {P2W18O62} and Cobalt Homobenzotrizoate for Supercapacitors and H2O2-Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CoK4[P2W18O62]
2.2. Synthesis of Co3(btc)2
2.3. Synthesis of CoK4[P2W18O62]@Co-BTC
3. Results and Discussion
3.1. Characterization
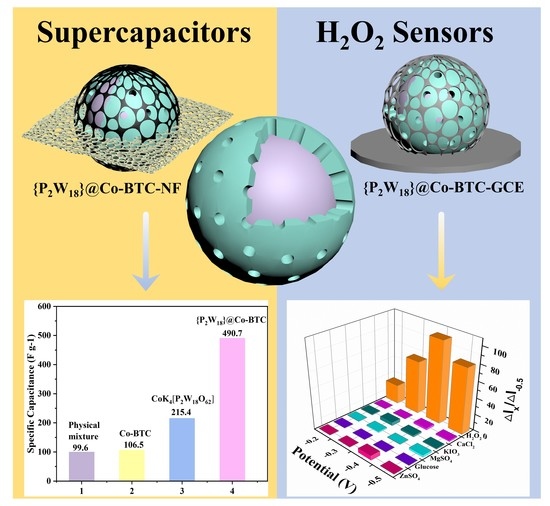
3.2. Supercapacitors Performence
3.3. H2O2 Sensor Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Z.; Wang, Y.; Cheng, T.; Yao, L.Q.; Li, X.; Lai, W.Y.; Huang, W. Printed supercapacitors: Materials, printing and applications. Chem. Soc. Rev. 2019, 48, 3229–3264. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.E.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chang, Z.H.; Wang, X.L.; Lin, H.Y.; Zhang, Y.C.; Liu, Q.Q.; Chen, Y.Z. Pseudocapacitance improvement of polymolybdates-based metal-organic complexes via modification with hydrogen molybdenum bronze by electrochemical treatment. Chem. Eng. J. 2022, 428, 132380. [Google Scholar] [CrossRef]
- Mustaqeem, M.; Naikoo, G.A.; Yarmohammadi, M.; Pedram, M.Z.; Pourfarzad, H.; Dar, R.A.; Taha, S.A.; Hassan, I.U.; Bhat, M.Y.; Chen, Y.F. Rational design of metal oxide based electrode materials for high performance supercapacitors—A review. J. Energy Storge 2022, 55, 105419. [Google Scholar] [CrossRef]
- Peng, Z.K.; Liu, X.; Meng, H.; Li, Z.J.; Li, B.J.; Liu, Z.Y.; Liu, S.C. Design and Tailoring of the 3D Macroporous Hydrous RuO2 Hierarchical Architectures with a Hard-Template Method for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 4577–4586. [Google Scholar] [CrossRef] [PubMed]
- Bagal, I.V.; Chodankar, N.R.; Waseem, A.; Johar, M.A.; Patil, S.J.; Abdullah, A.; Hassan, M.A.; Han, Y.K.; Ryu, S.W. CF4 plasma-treated porous silicon nanowire arrays laminated with MnO2 nanoflakes for asymmetric pseudocapacitors. Chem. Eng. J. 2021, 419, 129515. [Google Scholar] [CrossRef]
- Qiu, M.J.; Sun, P.; Shen, L.X.; Wang, K.; Song, S.Q.; Yu, X.; Tan, S.Z.; Zhao, C.X.; Mai, W.J. WO3 nanoflowers with excellent pseudo-capacitive performance and the capacitance contribution analysis. J. Mater. Chem. A 2016, 4, 7266–7273. [Google Scholar] [CrossRef]
- Mahmood, Q.; Kim, W.S.; Park, H.S. Structure and compositional control of MoO3 hybrids assembled by nanoribbons for improved pseudocapacitor rate and cycle performance. Nanoscale 2012, 4, 7855–7860. [Google Scholar] [CrossRef]
- Barbieri, O.; Hahn, M.; Herzog, A.; Kotz, R. Capacitance limits of high surface area activated carbons for double layer capacitors. Carbon 2005, 43, 1303–1310. [Google Scholar] [CrossRef]
- Liu, T.Y.; Liu, G.L. Block copolymer-based porous carbons for supercapacitors. J. Mater. Chem. A 2019, 7, 23476–23488. [Google Scholar] [CrossRef]
- Lobato, B.; Suárez, L.; Guardia, L.; Centeno, T.A. Capacitance and surface of carbons in supercapacitors. Carbon 2017, 122, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.P.; Yu, K.; Lv, J.H.; Guo, C.H.; Zhou, B.B. 3D POMOF based on {AsW12} cluster and Ag-MOF with interpenetrating channels for large-capacity aqueous asymmetric supercapacitors and highly selective biosensors detecting hydrogen peroxide. J. Mater. Chem. A 2020, 8, 22918–22928. [Google Scholar] [CrossRef]
- Yang, K.; Ying, Y.X.; Cui, L.L.; Sun, J.C.; Luo, H.; Hu, Y.Y.; Zhao, J.W. Stable aqueous Zn−Ag and Zn−polyoxometalate hybrid battery driven by successive Ag+ cation and polyoxoanion redox reactions. Energy Storage Mater. 2021, 34, 203–210. [Google Scholar] [CrossRef]
- Liu, J.C.; Wang, J.F.; Han, Q.; Guan, P.S.; Liu, L.L.; Chen, L.J.; Zhao, J.W.; Streb, C.; Song, Y.F. Multicomponent Self-Assembly of a Giant Heterometallic Polyoxotungstate Supercluster with Antitumor Activity. Angew. Chem. Int. Ed. 2021, 60, 11153–11157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Hemesh, A.; Biendicho, J.J.; Soria, L.M.; Garcia, D.R.; Morante, J.R.; Ballesteros, B.; Romero, P.G. Rational design of MXene/activated carbon/polyoxometalate triple hybrid electrodes with enhanced capacitance for organic-electrolyte supercapacitors. J. Colloid Interface Sci. 2022, 623, 947–961. [Google Scholar] [CrossRef]
- Hou, Y.; Chai, D.F.; Li, B.N.; Pang, H.J.; Ma, H.Y.; Wang, X.M.; Tan, L.C. Polyoxometalate-Incorporated Metallacalixarene@Graphene Composite Electrodes for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 20845–20853. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Lin, J.F.; Wang, C.Y.; Wang, X.L. Capped Keggin Type Polyoxometalate-Based Inorganic–Organic Hybrids Involving In Situ Ligand Transformation as Supercapacitors and Efficient Electrochemical Sensors for Detecting Cr(VI). Inorg. Chem. 2021, 60, 19287–19296. [Google Scholar] [CrossRef]
- Zhu, J.J.; Vilaua, R.B.; Romero, P.G. Can polyoxometalates enhance the capacitance and energy density of activated carbon in organic electrolyte supercapacitors? Electrochim. Acta 2020, 362, 137007. [Google Scholar] [CrossRef]
- Roy, S.; Vemuri, V.; Maiti, S.; Manoj, K.S.; Subbarao, U.; Peter, S.C. Two Keggin-Based Isostructural POMOF Hybrids: Synthesis, Crystal Structure, and Catalytic Properties. Inorg. Chem. 2018, 57, 12078–12092. [Google Scholar] [CrossRef]
- Yang, M.H.; Choi, B.G.; Jung, S.C.; Han, Y.K.; Huh, Y.S.; Lee, S.B. Polyoxometalate-coupled Graphene via Polymeric Ionic Liquid Linker for Supercapacitors. Adv. Funct. Mater. 2014, 24, 7301–7309. [Google Scholar] [CrossRef]
- Du, N.N.; Gong, L.G.; Fan, L.Y.; Yu, K.; Luo, H.; Pang, S.J.; Gao, J.Q.; Zheng, Z.W.; Lv, J.H.; Zhou, B.B. Nanocomposites Containing Keggin Anions Anchored on Pyrazine-Based Frameworks for Use as Supercapacitors and Photocatalysts. ACS Appl. Nano Mater. 2019, 2, 3039–3049. [Google Scholar] [CrossRef]
- Wang, G.N.; Chen, T.T.; Li, S.B.; Pang, H.J.; Ma, H.Y. A coordination polymer based on dinuclear (pyrazinyl tetrazolate) copper(ii) cations and Wells–Dawson anions for high-performance supercapacitor electrodes. Dalton Trans. 2017, 46, 13897–13902. [Google Scholar] [CrossRef]
- Fang, P.T.; Lu, X.M.; Zhou, Q.; Yan, D.X.; Xin, J.Y.; Xu, J.L.; Shi, C.Y.; Zhou, Y.Q.; Xia, S.Q. Controlled alcoholysis of PET to obtain oligomers for the preparation of PET-PLA copolymer. Chem. Eng. J. 2023, 451, 138988. [Google Scholar] [CrossRef]
- Hong, B.; Liu, L.; Wang, S.M.; Han, Z.B. Facile Synthesis of ZIF-8/ZnO/Polyoxometalate Ternary Composite Materials for Efficient and Rapid Removal of Cationic Organic Dye. J. Clust. Sci. 2016, 27, 563–571. [Google Scholar] [CrossRef]
- Li, J.P.; Wu, D.; Wang, C.L.; Liu, D.; Chen, W.L.; Wang, X.L.; Wang, E.B. Interfacial self-assembly engineering for constructing a 2D flexible superlattice polyoxometalate/rGO heterojunction for high-performance photovoltaic devices. Dalton Trans. 2020, 49, 3766–3774. [Google Scholar] [CrossRef]
- Yang, A.S.; Cui, L.P.; Yu, K.; Lv, J.H.; Ma, Y.J.; Zhao, T.T.; Zhou, B.B. Supramolecular Host−Guest Assembly Based on Phosphotungstate Nanostructures for Pseudocapacitive and Electrochemical Sensing Applications. ACS Appl. Nano Mater. 2022, 5, 10452–10461. [Google Scholar] [CrossRef]
- He, L.L.; Cui, L.P.; Yu, K.; Lv, J.H.; Ma, Y.J.; Tian, R.; Zhou, B.B. The pseudocapacitance and sensing materials constructed by Dawson/basket-like phosphomolybdate. J. Solid State Chem. 2022, 316, 123578. [Google Scholar] [CrossRef]
- Tian, R.; Cui, L.P.; Yu, K.; Lv, J.H.; Ma, Y.J.; He, L.L.; Zhou, B.B. Arsenotungstate-Nanostructure-Based Derivatives with One-Dimensional Tunnels for Electrochemical Capacitors and Electrocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 14882–14892. [Google Scholar] [CrossRef]
- Zhong, R.; Cui, L.P.; Yu, K.; Lv, J.H.; Guo, Y.H.; Zhang, E.M.; Zhou, B.B. Wells−Dawson Arsenotungstate Porous Derivatives for Electrochemical Supercapacitor Electrodes and Electrocatalytically Active Materials. Inorg. Chem. 2021, 60, 9869–9879. [Google Scholar] [CrossRef]
- Gao, J.Q.; Gong, L.G.; Fan, X.Y.; Yu, K.; Zheng, Z.W.; Zhou, B.B. {P2W18O62}-Encapsulated Potassium-Ion Nanotubes Interca-lated in Copper Biimidazole Frameworks for Supercapacitors and Hydrogen Peroxide Sensing. ACS Appl. Nano Mater. 2020, 3, 1497–1503. [Google Scholar] [CrossRef]
- Fonseca, J.; Gong, T.H.; Jiao, L.; Jian, H.L. Metal–organic frameworks (MOFs) beyond crystallinity: Amorphous MOFs, MOF liquids and MOF glasses. J. Mater. Chem. A 2021, 9, 10562. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.J.; Horn, Y.S.; Dinc, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Yang, X.Y.; Li, M.T.; Sheng, N.; Li, J.S.; Liu, G.D.; Sha, J.Q.; Jiang, J.Z. Structure and LIBs Anode Material Application of Novel Wells–Dawson Polyoxometalate-Based Metal Organic Frameworks with Different Helical Channels. Cryst. Growth Des. 2018, 18, 5564–5572. [Google Scholar] [CrossRef]
- Lü, Y.; Xiao, L.N.; Hao, X.R.; Cui, X.B.; Xu, J.Q. A series of organic–inorganic hybrid compounds formed by [P2W18O62]6− and several types of transition metal complexes. Dalton Trans. 2017, 46, 14393–14405. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.Y.; Guo, H.; Lv, J.H.; Yu, K.; Su, Z.H.; Wang, L.; Wang, C.M.; Zhou, B.B. Efficient and robust photocatalysts based on {P2W18} modified by Ag complex. Dalton Trans. 2018, 47, 4273–4281. [Google Scholar] [CrossRef]
- Cong, B.W.; Su, Z.H.; Zhao, Z.F.; Wang, B. A novel 3D POMOFs based on Wells-Dawson arsenomolybdates with excellent photocatalytic and lithium-ion batteries performance. CrystEngComm 2017, 19, 7154–7161. [Google Scholar] [CrossRef]
- Qin, Y.; Kong, X.G.; Lei, D.Q.; Lei, X.D. Facial Grinding Method for Synthesis of High-Purity CuS Nanosheets. Ind. Eng. Chem. Res. 2018, 57, 2759–2764. [Google Scholar] [CrossRef]
- Liang, Y.; Di, S.; Wang, C.M.; Yu, K.; Wang, C.X.; Lv, J.H.; Zhou, B.B. Synthesis of {P2W18}-based coated structured nano materials with supercapacitors and H2O2 sensing. J. Energy Storage 2022, 56, 105991. [Google Scholar] [CrossRef]
- Liang, C.Y.; Wang, X.; Yu, D.X.; Guo, W.; Zhang, F.; Qu, F.Y. In-situ immobilization of a polyoxometalate metal-organic framework (NENU-3) on functionalized reduced graphene oxide for hydrazine sensing. Chin. J. Chem. 2021, 39, 2889–2897. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Z.X.; Miras, H.N.; Song, Y.F. Modular Polyoxometalate-Layered Double Hydroxide Composites as Efficient Oxidative Catalysts. Chem. Eur. J. 2015, 21, 10812–10820. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.S.; Bai, L.L.; Xiong, W.W.; Li, P.Z.; Ding, J.F.; Zhang, G.D.; Wu, T.; Zhao, Y.L.; Lee, J.M.; Yang, Y.H.; et al. Surfactant Media To Grow New Crystalline Cobalt 1,3,5-Benzenetricarboxylate Metal−Organic Frameworks. Inorg. Chem. 2014, 53, 8529–8537. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Wan, J.Q.; Ma, Y.W.; Wang, Y.; Chen, X.; Guan, Z.Y. Degradation of refractory dibutyl phthalate by peroxymonosulfate activated with novel catalysts cobalt metal-organic frameworks: Mechanism, performance, and stability. J. Hazard. Mater. 2016, 318, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Duan, X.X.; Zhao, J.; Wang, X.L.; Jiang, Z.J. Homogeneous borotungstic acid and heterogeneous micellar borotungstic acid catalysts for biodiesel production by esterification of free fatty acid. Biomass Bioenergy 2015, 76, 31–42. [Google Scholar] [CrossRef]
- Lü, Y.Y.; Zhan, W.W.; He, Y.; Wang, Y.T.; Kong, X.J.; Kuang, Q.; Xie, Z.X.; Zheng, L.S. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar] [CrossRef]
- Chae, I.S.; Kim, M.; Kang, Y.S.; Kang, S.W. Enhanced CO2 carrier activity of potassium cation with fluorosilicate anions for facilitated transport membranes. J. Membr. Sci. 2014, 466, 357–360. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Zhang, J.; Yang, Y.L.; Wang, J.Q.; Hu, B.Y.; Wang, W.; Cao, W.; Gai, S.; Xia, D.B.; Lin, K.F.; et al. Multifunctional nanostructured host-guest POM@MOF with lead sequestration capability induced stable and efficient perovskite solar cells. Nano Energy 2022, 97, 107184. [Google Scholar] [CrossRef]
- Shreyanka, S.N.; Theerthagiri, J.; Lee, S.J.; Yu, Y.; Choi, M.Y. Multiscale design of 3D metal–organic frameworks (M−BTC, M: Cu, Co, Ni) via PLAL enabling bifunctional electrocatalysts for robust overall water splitting. Chem. Eng. J. 2022, 446, 137045. [Google Scholar] [CrossRef]
- Tang, Y.J.; Chen, Y.F.; Zhu, H.J.; Zhang, A.M.; Wang, X.L.; Dong, L.Z.; Li, S.L.; Xu, Q.; Lan, Y.Q. Solid-Phase Hot-Pressing Synthesis of POMOFs on Carbon Cloth and Derived Phosphides for All pH Values Hydrogen Evolution. J. Mater. Chem. A 2018, 6, 21969–21977. [Google Scholar] [CrossRef]
- Li, C.; Lou, X.B.; Shen, M.; Hu, X.S.; Guo, Z.; Wang, Y.; Hu, B.W.; Chen, Q. High Anodic Performance of Co 1,3,5-Benzenetricarboxylate Coordination Polymers for Li-Ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 15352–15360. [Google Scholar] [CrossRef]
- Wang, L.Y.; Kang, N.; Gong, L.G.; Wang, C.X.; Yu, K.; Wang, C.M.; Zhou, B.B. A novel core-shell structured hybrid composed of zinc homobenzotrizoate and silver borotungstate with supercapacitor and photocatalytic dye degradation performance. J. Energy Storage 2022, 46, 103873. [Google Scholar] [CrossRef]
- Zhong, X.H.; Lu, Y.; Luo, F.; Liu, Y.W.; Li, X.H.; Liu, S.X. A nanocrystalline POM@MOFs catalyst for the degradation of phenol: Effective cooperative catalysis by metal nodes and POM guests. Chem. Eur. J. 2018, 24, 3045–3051. [Google Scholar] [CrossRef]
- Gautam, J.; Liu, Y.; Gu, J.; Ma, Z.Y.; Dahal, B.; Chishti, A.N.; Ni, L.B.; Diao, G.W.; Wei, Y.G. Three-dimensional nano assembly of nickel cobalt sulphide/polyaniline@polyoxometalate/reduced graphene oxide hybrid with superior lithium storage and electrocatalytic properties for hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 614, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.F.; Garcíab, C.J.G.; Li, B.N.; Pang, H.J.; Ma, H.Y.; Wang, X.M.; Tan, L.C. Polyoxometalate-based metal-organic frameworks for boosting electrochemical capacitor performance. Chem. Eng. J. 2019, 373, 587–597. [Google Scholar] [CrossRef]
- Yang, K.; Hu, Y.Y.; Li, L.Y.; Cui, L.L.; He, L.; Wang, S.J.; Zhao, J.W.; Song, Y.F. First high-nuclearity mixed-valence polyoxometalate with hierarchical interconnected Zn2+ migration channels as an advanced cathode material in aqueous zinc-ion battery. Nano Energy 2020, 74, 104851. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Santos, C.S.; Garcia, J.R.; Vidotti, M.; Marchesi, L.F.; Pessoa, C.A. IR drop studies of poly(aniline)-based modified electrodes. J. Electroanal. Chem. 2020, 878, 114662. [Google Scholar] [CrossRef]
- Wang, D.W.; Wang, Y.T.; Liu, H.W.; Xu, W.; Xu, L. Unusual carbon nanomesh constructed by interconnected carbon nanocages for ionic liquid-based supercapacitor with superior rate capability. Chem. Eng. J. 2018, 342, 474–483. [Google Scholar] [CrossRef]
- Boussema, F.; Grossa, A.J.; Hmida, F.; Ayed, B.; Majdoub, H.; Cosnier, S.; Maaref, A.; Holzinger, M. Dawson-type polyoxometalate nanoclusters confined in a carbon nanotube matrix as efficient redox mediators for enzymatic glucose biofuel cell anodes and glucose biosensors. Biosens. Bioelectron. 2018, 109, 20–26. [Google Scholar] [CrossRef]
- Shinde, P.A.; Khan, M.F.; Rehman, M.A.; Jung, E.; Pham, Q.N.; Won, Y.; Jun, S.C. Nitrogen-doped carbon integrated nickel–cobalt metal phosphide marigold flowers as a high capacity electrode for hybrid supercapacitors. CrystEngComm 2020, 22, 6360. [Google Scholar] [CrossRef]
- Li, X.; Rong, J.P.; Wei, B.Q. Electrochemical behavior of single-walled carbon nanotube supercapacitors under compressive stress. ACS Nano 2010, 4, 6039–6049. [Google Scholar] [CrossRef]
- Qiu, Y.F.; Dai, X.F.; Wang, Y.P.; Ji, X.Y.; Ma, Z.; Liu, S.Q. The polyoxometalates mediated preparation of phosphate-modified NiMoO4-x with abundant O-vacancies for H2 production via urea electrolysis. J. Colloid Interface Sci. 2023, 629, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lei, S.J.; Tu, Q.Y.; Rao, L.H.; Zen, W.H.; Xiao, Y.H.; Cheng, B.C. Phase-controlled growth of nickel hydroxide nanostructures on nickel foam for enhanced supercapacitor performance. J. Energy Storage 2021, 43, 103171. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Ammam, M. A molecular hybrid polyoxometalate-organometallic moieties and its relevance to supercapacitors in physiological electrolytes Selvaraj Chinnathambi. J. Power Source 2015, 284, 524–535. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Sun, C.L.; Wu, Z.S. Room-temperature fast assembly of 3D macroscopically porous graphene frameworks for binder-free compact supercapacitors with high gravimetric and volumetric capacitances. J. Energy Chem. 2021, 61, 23–28. [Google Scholar] [CrossRef]
- Skunik, M.; Chojak, M.; Rutkowska, I.A.; Kulesza, P.J. Improved capacitance characteristics during electrochemical charging of carbon nanotubes modified with polyoxometallate monolayers. Electrochim. Acta 2008, 53, 3862–3869. [Google Scholar] [CrossRef]
- Zheng, Z.W.; Zhao, X.Y.; Gong, L.G.; Wang, C.X.; Wang, C.M.; Yu, K.; Zhou, B.B. Coral-like {SiW10Mn2}-based Mn-MOFs: Facile fabrication with high electrochemical capacitor performance. J. Solid State Chem. 2020, 288, 121409. [Google Scholar] [CrossRef]
- Fu, D.Y.; Li, H.W.; Zhang, X.M.; Han, G.Y.; Zhou, H.H.; Chang, Y.Z. Flexible solid-state supercapacitor fabricated by metal-organic framework/graphene oxide hybrid interconnected with PEDOT. Mater. Chem. Phys. 2016, 179, 166–173. [Google Scholar] [CrossRef]
- Miao, F.J.; Shao, C.L.; Li, X.H.; Wang, K.X.; Lu, N.; Liu, Y.C. Three-dimensional freestanding hierarchically porous carbon materials as binder-free electrodes for supercapacitors: High capacitive property and long-term cycling stability. J. Mater. Chem. A 2016, 4, 5623–5631. [Google Scholar] [CrossRef]
- Li, W.H.; Ding, K.; Tian, H.R.; Yao, M.S.; Nath, B.; Deng, W.H.; Wang, Y.B.; Xu, G. Conductive Metal–Organic Framework Nanowire Array Electrodes for High-Performance Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhao, X.Y.; Wang, C.M.; Yu, K.; Lv, J.H.; Wang, C.X.; Zhou, B.B. The supercapacitor and photocatalytic supermolecule materials constructed by 4′4-pyridine and {PMo12O40}. J. Solid State Chem. 2022, 312, 123235. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, N.; Wang, C.M.; Yu, K.; Lv, J.H.; Wang, C.X.; Zhou, B.B. A hybrid of borotungstate-coated metal-organic framework with supercapacitance, photocatalytic dye degradation and H2O2 sensing properties. Dalton Trans. 2022, 51, 7613–7621. [Google Scholar]
- Li, X.; Sun, L.J.; Yang, X.Y.; Zhou, K.F.; Zhang, G.G.; Tong, Z.B.; Wang, C.; Sha, J.Q. Enhancing colorimetric detection of H2O2 and ascorbic acid on polypyrrole coating fluconazole functionalized POMOFs. Analyst 2019, 144, 3347–3356. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, S.; Sun, C.G. Ni3Mo3N coupled with nitrogen-rich carbon microspheres as an efficient hydrogen evolution reaction catalyst and electrochemical sensor for H2O2 detection. Int. J. Hydrogen Energy 2022, 47, 14906–14915. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhang, X.M.; Chai, X.Y.; Wang, T.Q.; Cao, T.L.; Li, Y.N.; Zhang, L.Y.; Fan, F.Q.; Fu, Y.; Qi, W. An Electrochemical Sensor for H2O2 Based on Au Nanoparticles Embedded in UiO-66 Metal–Organic Framework Films. ACS Appl. Nano Mater. 2021, 4, 6103–6110. [Google Scholar] [CrossRef]
- Li, W.W.; Liu, J.; Chen, C.; Zhu, Y.D.; Liu, N.; Zhou, Y.M.; Chen, S.R. High catalytic performance non-enzymatic H2O2 sensor based on Cu2O@Cu9S5 yolk-shell nanospheres. Appl. Surf. Sci. 2022, 587, 152766. [Google Scholar] [CrossRef]
- Sun, C.X.; Ying, J.; Zhang, Y.P.; Jin, L.; Tian, A.X.; Wang, X.L. A series of POM-based compounds by tunning coordination groups and spacers of ligands: Electrocatalytic, capacitive and photoelectrocatalytic properties. CrystEngComm 2022, 24, 587–600. [Google Scholar] [CrossRef]
- Jin, L.; Ying, J.; Zhang, Y.P.; Sun, C.X.; Tian, A.X.; Wang, X.L. A series of polyoxometalate compounds by tuning N sites and numbers of ligands: Syntheses, characterization and electrochemical sensing, and photocatalytic and supercapacitor properties. New J. Chem. 2022, 46, 8422. [Google Scholar] [CrossRef]
- Zhu, H.T.; Du, J.; Lu, Y.; Su, F.; Li, Y.G. Immobilization of enzymes on an organic–inorganic hybrid network consisting of Dawson-type polyoxotungstate and a zinc(II)-biimidazole complex moiety. New J. Chem. 2019, 43, 146–153. [Google Scholar] [CrossRef]
- Kong, X.X.; Shen, Q.Q.; Wan, T.T.; Li, K.Y.; Sun, F.G.; Wu, H.L. Two silver(I) complexes: Synthesis, structures, and electrochemical H2O2 sensing. J. Chin. Chem. Soc. 2022, 69, 540–548. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Lu, J.J.; Lin, H.Y.; Wang, X.L.; Chang, Z.H.; Chen, Y.Z.; Zhang, Y.C. Polyoxometalate-based metal–organic complexes constructed from a new bispyrimidine-amide ligand with high capacitance performance and selectivity for the detection of Cr(VI). Chin. Chem. Lett. 2022, 33, 4389–4394. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zheng, J.B. Environmentally friendly synthesis of Co-based zeolitic imidazolate framework and its application as H2O2 sensor. Chem. Eng. J. 2020, 392, 123690. [Google Scholar] [CrossRef]
- Zhao, T.T.; Cui, L.P.; Yu, K.; Lv, J.H.; Ma, Y.J.; Yang, A.S.; Zhou, B.B. Porous {P6Mo18O73}-type Poly(oxometalate) Metal−Organic Frameworks for Improved Pseudocapacitance and Electrochemical Sensing Performance. ACS Appl. Mater. Interfaces 2022, 24, 30099–30111. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, F.B.; Li, S.B.; Zhang, L.; Sun, M. A review of application and prospect for polyoxometalate-based composites in electrochemical sensor. Inorg. Chem. Commun. 2022, 235, 109084. [Google Scholar] [CrossRef]
- Ammar, S.H.; Salman, M.D.; Shafi, R.F. Keggin- and Dawson-type polyoxotungstates immobilized on poly(3, 4-ethylenedioxythiophene)-coated zerovalent iron nanoparticles: Synthesis, characterization and their catalytic oxidative desulfurization activity. J. Environ. Chem. Eng. 2021, 9, 104904. [Google Scholar] [CrossRef]
- Shi, N.; Liu, D.; Dang, F.L.; Chen, Q.T.; Li, M.; Wen, F.S. Bifunctional and recyclable Dawson-type polyoxometalates catalyze oxidative degradation of lignocellulose to selectively produce phthalates. Bioresource Technol. 2019, 273, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.P.; Yang, G.C.; Jia, Y.T.; Gong, J.; Su, Z.M.; Qu, L.Y. ITO electrode modified with chitosan nanofibers loading polyoxometalate by one step self-assembly method and its electrocatalysis. Electrochem. Commun. 2007, 9, 2224–2228. [Google Scholar] [CrossRef]
- Chi, Y.N.; Cui, F.Y.; Lin, Z.G.; Xu, Y.; Ma, X.Y.; Shen, P.P.; Huang, K.L.; Hu, C.W. Assembly of Cu/Ag-quinoxaline-polyoxotungstate hybrids: Influence of Keggin and Wells–Dawson polyanions on the structure. J. Solid State Chem. 2013, 199, 230–239. [Google Scholar] [CrossRef]
- Wang, R.X.; Liu, Y.F.; Zuo, P.; Zhang, Z.D.; Lei, N.N.; Liu, Y.Q. Phthalocyanine-sensitized evolution of hydrogen and degradation of organic pollutants using polyoxometalate photocatalysts. Environ. Sci. Pollut. Res. 2020, 27, 18831–18842. [Google Scholar] [CrossRef]
- Zheng, X.T.; Chen, W.L.; Chen, L.; Wang, Y.J.; Guo, X.W.; Wang, J.B.; Wang, E.B. A strategy for breaking polyoxometalate-based MOFs to obtain high loading amount of nanosized polyoxometalate clusters to improve the performance of dye-sensitized solar cells. Chem. Eur. J. 2017, 23, 8871–8878. [Google Scholar] [CrossRef]
- Liu, S.P.; Xu, L.; Li, F.Y.; Guo, W.H.; Xing, Y.; Sun, Z.X. Carbon nanotubes-assisted polyoxometalate nanocomposite film with enhanced electrochromic performance. Electrochim. Acta 2011, 56, 8156–8162. [Google Scholar] [CrossRef]
- Xing, R.M.; Tong, L.Y.; Zhao, X.Y.; Liu, H.L.; Ma, P.T.; Zhao, J.W.; Liu, X.Q.; Liu, S.H. Rapid and sensitive electrochemical detection of myricetin based on polyoxometalates/SnO2/gold nanoparticles ternary nanocomposite film electrode. Sens. Actuators B Chem. 2019, 283, 35–41. [Google Scholar] [CrossRef]
- Mu, A.Q.; Li, J.S.; Chen, W.L.; Sang, X.J.; Su, Z.M.; Wang, E.B. The composite material based on Dawson-type polyoxometalate and activated carbon as the supercapacitor electrode. Inorg. Chem. Commun. 2015, 55, 149–152. [Google Scholar] [CrossRef]
- Guan, Y.; Cui, L.P.; Yu, K.; Lv, J.H.; Deng, Y.F.; Wang, C.M.; Zhou, B.B. Two arsenic capped Dawson-type supramolecular hybrid assemblies induced by benzimidazole for photo-/electro-catalytic performance. J. Solid State Chem. 2020, 292, 121707. [Google Scholar] [CrossRef]
- Hou, Y.; Pang, H.J.; Gómez-García, C.J.; Ma, H.Y.; Wang, X.M.; Tan, L.C. Polyoxometalate Metal−Organic Frameworks: Keggin Clusters Encapsulated into Silver-Triazole Nanocages and Open Frameworks with Supercapacitor Performance. Inorg. Chem. 2019, 58, 16028–16039. [Google Scholar] [CrossRef]
- Chai, D.F.; Hou, Y.; O’Halloran, K.P.; Pang, H.J.; Ma, H.Y.; Wang, G.N.; Wang, X.M. Enhancing Energy Storage via TEA-Dependent Controlled Syntheses: Two Series of Polyoxometalate-Based Inorganic-Organic Hybrids and their Supercapacitor Properties. Chem. Electro. Chem. 2018, 5, 3443–3450. [Google Scholar] [CrossRef]
- Gallegos, A.K.C.; Cantú, M.L.; Pastor, N.C.; Romero, P.G. Nanocomposite Hybrid Molecular Materials for Application in Solid-State Electrochemical Supercapacitors. Adv. Funct. Mater. 2015, 15, 1125–1133. [Google Scholar] [CrossRef]
- Guevara, J.S.; Ruiza, V.; Romero, P.G. Stable graphene–polyoxometalate nanomaterials for application in hybrid supercapacitors. Phys. Chem. Chem. Phys. 2014, 16, 20411–20414. [Google Scholar] [CrossRef]
- Hu, C.C.; Zhao, E.B.; Nitta, N.; Magasinski, A.; Berdichevsky, G.; Yushin, G. Aqueous solutions of acidic ionic liquids for enhanced stability of polyoxometalate-carbon supercapacitor electrodes. J. Power Sources 2016, 326, 569–574. [Google Scholar] [CrossRef]
- Genovese, M.; Lian, K. Polyoxometalate modified pine cone biochar carbon for supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3939–3947. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Vinu, A.; Kim, D.H.; Gomez-Romero, P. Asymmetric Supercapacitors Based on Reduced Graphene Oxide with Different Polyoxometalates as Positive and Negative Electrodes. ChemSusChem 2017, 10, 2742–2750. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Ramadan, M.; Ahmed, N.; ElNaga, A.O.A.; Alalawy, H.H.; Zaki, T.; Shaban, S.A.; Hassan, H.B.; Allam, N.K. Metal–Organic frameworks encapsulated with vanadium-substituted heteropoly acid for highly stable asymmetric supercapacitors. J. Energy Storage 2020, 28, 101292. [Google Scholar] [CrossRef]
- Li, T.Y.; He, P.; Dong, Y.N.; Chen, W.C.; Wang, T.; Gong, J.; Chen, W.L. Polyoxometalate-Based Metal-Organic Framework/Polypyrrole Composites toward Enhanced Supercapacitor Performance. Eur. J. Inorg. Chem. 2021, 21, 2063–2069. [Google Scholar] [CrossRef]
- Quezada, E.F.; Casillas, D.C.M.; Gallegos, A.K.C.; Llave, E.d.l. Effect of Hierarchical Porosity on PMo12 Adsorption and Capacitance in Hybrid Carbon–PMo12 Electrodes for Supercapacitors. Energy Fuels 2022, 36, 3987–3996. [Google Scholar] [CrossRef]
- Hu, S.M.; Li, K.Q.; Yu, X.J.; Jin, Z.X.; Xiao, B.X.; Yang, R.R.; Pang, H.J.; Ma, H.Y.; Wang, X.M.; Tan, L.C.; et al. Enhancing the electrochemical capacitor performance of Keggin polyoxometalates by anchoring cobalt-triazole complexes. J. Mol. Struct. 2022, 1250, 131753. [Google Scholar] [CrossRef]
- Hou, Y.J.; Han, P.L.; Zhang, L.K.; Li, H.; Xu, Z.H. pH-controlled assembling of POM-based metal–organic frameworks for use as supercapacitors andefficient oxidation catalysts for various sulfides. Inorg. Chem. Front. 2023, 10, 148–157. [Google Scholar] [CrossRef]
- Yu, X.J.; Khan, S.U.; Jin, Z.X.; Wu, Q.; Pang, H.J.; Ma, H.Y.; Wang, X.M.; Tan, L.C.; Yang, G.X. Water cluster encapsulated polyoxometalate-based hydrogen-bonded supramolecular frameworks (PHSFs) as a new family of high-capacity electrode materials. J. Energy Storage 2022, 53, 105192. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Di, S.; Lin, H.; Wang, C.; Yu, K.; Lv, J.; Wang, C.; Zhou, B. Nanomaterial with Core–Shell Structure Composed of {P2W18O62} and Cobalt Homobenzotrizoate for Supercapacitors and H2O2-Sensing Applications. Nanomaterials 2023, 13, 1176. https://doi.org/10.3390/nano13071176
Zhang L, Di S, Lin H, Wang C, Yu K, Lv J, Wang C, Zhou B. Nanomaterial with Core–Shell Structure Composed of {P2W18O62} and Cobalt Homobenzotrizoate for Supercapacitors and H2O2-Sensing Applications. Nanomaterials. 2023; 13(7):1176. https://doi.org/10.3390/nano13071176
Chicago/Turabian StyleZhang, Lanyue, Shan Di, Hong Lin, Chunmei Wang, Kai Yu, Jinghua Lv, Chunxiao Wang, and Baibin Zhou. 2023. "Nanomaterial with Core–Shell Structure Composed of {P2W18O62} and Cobalt Homobenzotrizoate for Supercapacitors and H2O2-Sensing Applications" Nanomaterials 13, no. 7: 1176. https://doi.org/10.3390/nano13071176