Enhanced Water Adsorption of MIL-101(Cr) by Metal-Organic Polyhedral Encapsulation for Adsorption Cooling
Abstract
:1. Introduction
2. Materials and Methods
2.1. MOP/MIL-101(Cr) Preparation
2.2. MOP/MIL-101(Cr) Characterization
2.3. Water Adsorption Rate Measurement
2.4. Mathematical Modeling of ACS
3. Results and Discussion
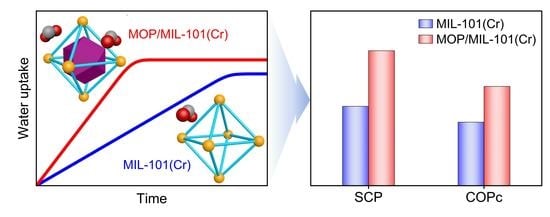
3.1. Effects of Structural Characteristics on Water Adsorption Rates
3.2. Adsorption Cooling Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawal, R.; Schweiker, M.; Kazanci, O.B.; Vardhan, V.; Jin, Q.; Duanmu, L. Personal Comfort Systems: A Review on Comfort, Energy, and Economics. Energ. Build. 2020, 214, 109858. [Google Scholar] [CrossRef]
- Meunier, F. Adsorption Heat Powered Heat Pumps. Appl. Therm. Eng. 2013, 61, 830–836. [Google Scholar] [CrossRef]
- Ziegler, F. State of the Art in Sorption Heat Pumping and Cooling Technologies. Int. J. Refrig. 2002, 25, 450–459. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Adsorptive Heat Storage and Amplification: New Cycles and Adsorbents. Energy 2019, 167, 440–453. [Google Scholar] [CrossRef]
- Aristov, Y.I. Optimal Adsorbent for Adsorptive Heat Transformers: Dynamic Considerations. Int. J. Refrig. 2009, 32, 675–686. [Google Scholar] [CrossRef]
- Critoph, R.E.; Zhong, Y. Review of Trends in Solid Sorption Refrigeration and Heat Pumping Technology. Proc. Inst. Mech. Eng. E J. Process. 2004, 219, 285–300. [Google Scholar] [CrossRef]
- Demir, H.; Mobedi, M.; Ülkü, S. A Review on Adsorption Heat Pump: Problems and Solutions. Renew. Sustain. Energ. Rev. 2008, 12, 2381–2403. [Google Scholar] [CrossRef] [Green Version]
- Aristov, Y.I. Challenging Offers of Material Science for Adsorption Heat Transformation: A Review. Appl. Therm. Eng. 2013, 50, 1610–1618. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- De Lange, M.F.; Verouden, K.J.; Vlugt, T.J.; Gascon, J.; Kapteijn, F. Adsorption-Driven Heat Pumps: The Potential of Metal-Organic Frameworks. Chem. Rev. 2015, 115, 12205–12250. [Google Scholar] [CrossRef]
- Furukawa, H.; Gandara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal-Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Férey, C.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal-Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Sun, B.; Chakraborty, A. Thermodynamic Frameworks of Adsorption Kinetics Modeling: Dynamic Water Uptakes on Silica Gel for Adsorption Cooling Applications. Energy 2015, 84, 296–302. [Google Scholar] [CrossRef]
- Lee, J.G.; Bae, K.J.; Kwon, O.K. Performance Investigation of a Two-Bed Type Adsorption Chiller with Various Adsorbents. Energies 2020, 13, 2553. [Google Scholar] [CrossRef]
- Rupam, T.H.; Tuli, F.J.; Jahan, I.; Palash, M.L.; Chakraborty, A.; Saha, B.B. Isotherms and Kinetics of Water Sorption onto MOFs for Adsorption Cooling Applications. Therm. Sci. Eng. Prog. 2022, 34, 101436. [Google Scholar] [CrossRef]
- Muttakin, M.; Pal, A.; Rupa, M.J.; Ito, K.; Saha, B.B. A Critical Overview of Adsorption Kinetics for Cooling and Refrigeration Systems. Adv. Colloid Interface Sci. 2021, 294, 102468. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Experimental Investigation for Water Adsorption Characteristics on Functionalized MIL-125(Ti) MOFs: Enhanced Water Transfer and Kinetics for Heat Transformation Systems. Int. J. Heat Mass Transf. 2022, 186, 122473. [Google Scholar] [CrossRef]
- Han, B.; Chakraborty, A. Advanced Cooling Heat Pump and Desalination Employing Functional UiO-66(Zr) Metal-Organic Frameworks. Energ. Convers. Manag. 2020, 213, 112825. [Google Scholar] [CrossRef]
- Islamoglu, T.; Goswami, S.; Li, Z.; Howarth, A.J.; Farha, O.K.; Hupp, J.T. Postsynthetic Tuning of Metal-Organic Frameworks for Targeted Applications. Acc. Chem. Res. 2017, 50, 805–813. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal-Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-Synthetic Metalation of Metal-organic Frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Morabito, J.V.; Tsung, C.K. Core–Shell Catalysts of Metal Nanoparticle Core and Metal-Organic Framework Shell. ACS Catal. 2014, 4, 4409–4419. [Google Scholar] [CrossRef]
- Fang, Y.; Li, J.; Togo, T.; Jin, F.; Xiao, Z.; Liu, L.; Drake, H.; Lian, X.; Zhou, H.C. Ultra-Small Face-Centered-Cubic Ru Nanoparticles Confined within a Porous Coordination Cage for Dehydrogenation. Chem 2018, 4, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Kinik, F.P.; Uzun, A.; Keskin, S. Ionic Liquid/Metal-Organic Framework Composites: From Synthesis to Applications. ChemSusChem 2017, 10, 2842–2863. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Ni, Z.; O’Keeffe, M.; Yaghi, O.M. Reticular Chemistry of Metal-Organic Polyhedra. Angew. Chem. Int. Ed. Engl. 2008, 47, 5136–5147. [Google Scholar] [CrossRef]
- Gosselin, A.J.; Rowland, C.A.; Bloch, E.D. Permanently Microporous Metal-Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014. [Google Scholar] [CrossRef]
- Lee, J.; Lim, D.W.; Dekura, S.; Kitagawa, H.; Choe, W. MOP × MOF: Collaborative Combination of Metal-Organic Polyhedra and Metal-Organic Framework for Proton Conductivity. ACS Appl. Mater. Interfaces 2019, 11, 12639–12646. [Google Scholar] [CrossRef]
- Qiu, X.; Zhong, W.; Bai, C.; Li, Y. Encapsulation of a Metal-Organic Polyhedral in the Pores of a Metal-Organic Framework. J. Am. Chem. Soc. 2016, 138, 1138–1141. [Google Scholar] [CrossRef]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hou, Y.; Li, X.; Lü, H.; Hou, H. 1-D Helical Co(II) Metal-Organic Polymer: Synthesis, Structure, and Fluorescent Property. Synth. React. Inorg. Met. 2010, 40, 893–898. [Google Scholar] [CrossRef]
- Rallapalli, P.B.S.; Raj, M.C.; Senthilkumar, S.; Somani, R.S.; Bajaj, H.C. HF-Free Synthesis of MIL-101(Cr) and Its Hydrogen Adsorption Studies. Environ. Prog. Sustain. 2016, 35, 461–468. [Google Scholar] [CrossRef]
- Makhanya, N.; Oboirien, B.; Ren, J.; Musyoka, N.; Sciacovelli, A. Recent Advances on Thermal Energy Storage Using Metal-Organic Frameworks (MOFs). J. Energy Storage 2021, 34, 102179. [Google Scholar] [CrossRef]
- Uyun, A.S.; Miyazaki, T.; Ueda, Y.; Akisawa, A. High Performance Cascading Adsorption Refrigeration Cycle with Internal Heat Recovery Driven by a Low Grade Heat Source Temperature. Energies 2009, 2, 1170–1191. [Google Scholar] [CrossRef]
- Saha, B.B.; El-Sharkawy, I.I.; Chakraborty, A.; Koyama, S. Study on an Activated Carbon Fiber-Ethanol Adsorption Chiller: Part I–System Description and Modelling. Int. J. Refrig. 2007, 30, 86–95. [Google Scholar] [CrossRef]
- De Lange, M.F.; van Velzen, B.L.; Ottevanger, C.P.; Verouden, K.J.; Lin, L.C.; Vlugt, T.J.; Gascon, J.; Kapteijn, F. Metal-Organic Frameworks in Adsorption-Driven Heat Pumps: The Potential of Alcohols as Working Fluids. Langmuir 2015, 31, 12783–12796. [Google Scholar] [CrossRef]
- Alahmer, A.; Ajib, S.; Wang, X. Comprehensive Strategies for Performance Improvement of Adsorption Air Conditioning Systems: A Review. Renew. Sust. Energ. Rev. 2019, 99, 138–158. [Google Scholar] [CrossRef]
- Saha, B.B.; El-Sharkawy, I.I.; Chakraborty, A.; Koyama, S. Study on an Activated Carbon Fiber-Ethanol Adsorption Chiller: Part II–Performance Evaluation. Int. J. Refrig. 2007, 30, 96–102. [Google Scholar] [CrossRef]
- Tan, K.; Nijem, N.; Canepa, P.; Gong, Q.; Li, J.; Thonhauser, T.; Chabal, Y.J. Stability and Hydrolyzation of Metal Organic Frameworks with Paddle-Wheel SBUs upon Hydration. Chem. Mater. 2012, 24, 3153–3167. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, A.; Prabakaran, V.; Zhao, D.; Chua, K.J. A Review of Metal-Organic Frameworks (MOFs) as Energy-Efficient Desiccants for Adsorption Driven Heat-Transformation Applications. Appl. Energ. 2020, 269, 115070. [Google Scholar] [CrossRef]
- Xia, X.; Li, S. Improved Adsorption Cooling Performance of MIL-101(Cr)/GO Composites by Tuning the Water Adsorption Rate. Sustain. Energ. Fuels 2023, 7, 437–447. [Google Scholar] [CrossRef]
- Wang, S.; Xia, X.; Li, S. Cooling Performance of Metal Organic Framework-Water Pairs in Cascaded Adsorption Chillers. Appl. Therm. Eng. 2021, 189, 116707. [Google Scholar] [CrossRef]
- Shi, B.; Al-Dadah, R.; Mahmoud, S.; Elsayed, A.; Elsayed, E. CPO-27(Ni) Metal-Organic Framework Based Adsorption System for Automotive Air Conditioning. Appl. Therm. Eng. 2016, 106, 325–333. [Google Scholar] [CrossRef]








| Adsorbents | Cp,ad (kJ/K∙kg) | qst (kJ/kg) |
|---|---|---|
| MIL-101(Cr) | 1.31 | 2603 |
| MOP/MIL-101(Cr)-5 | 1.53 | 2676 |
| MOP/MIL-101(Cr)-10 | 1.55 | 2815 |
| MOP/MIL-101(Cr)-15 | 1.58 | 2883 |
| MOP/MIL-101(Cr)-20 | 1.62 | 2954 |
| MOP/MIL-101(Cr)-35 | 1.66 | 3048 |
| Parameters | Symbol | Value |
|---|---|---|
| Adsorption temperature | Tads | 303 K |
| Evaporation temperature | Teva | 283 K |
| Condensation temperature | Tcon | 303 K |
| Desorption temperature | Tdes | 353 K |
| Hot water mass flow | mhot | 1.5 kg/s |
| Cooling water mass flow | mcooling | 1.5 kg/s |
| Chilled water mass flow | mchill | 1.0 kg/s |
| Operating time | to | 1000 s |
| Switch time | ts | 60 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Liu, B.; Zhao, B.; Xia, Z.; Li, S. Enhanced Water Adsorption of MIL-101(Cr) by Metal-Organic Polyhedral Encapsulation for Adsorption Cooling. Nanomaterials 2023, 13, 1147. https://doi.org/10.3390/nano13071147
Xia X, Liu B, Zhao B, Xia Z, Li S. Enhanced Water Adsorption of MIL-101(Cr) by Metal-Organic Polyhedral Encapsulation for Adsorption Cooling. Nanomaterials. 2023; 13(7):1147. https://doi.org/10.3390/nano13071147
Chicago/Turabian StyleXia, Xiaoxiao, Boyun Liu, Bo Zhao, Zichao Xia, and Song Li. 2023. "Enhanced Water Adsorption of MIL-101(Cr) by Metal-Organic Polyhedral Encapsulation for Adsorption Cooling" Nanomaterials 13, no. 7: 1147. https://doi.org/10.3390/nano13071147