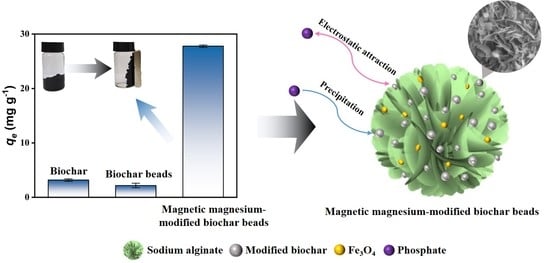
Recyclable Magnesium-Modified Biochar Beads for Efficient Removal of Phosphate from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnesium-Modified Biochar
2.3. Preparation of Bead Adsorbents
2.4. Characterizations
2.5. PO4-P Adsorption
2.6. Cyclic Performance Test
3. Results and Discussion
3.1. Pre-Screening the Conditions for Adsorbent Preparation
3.2. Adsorption Kinetics, Isotherms and Thermodynamics of the Bead Adsorbents
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherms
3.2.3. Adsorption Thermodynamics
3.3. Effect of pH Values on Adsorption Performance
3.4. Adsorption Mechanisms
3.5. Cyclic Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Fang, L.; Zeng, W.; Xu, L.; Huang, L.Z. Green rusts as a new solution to sequester and stabilize phosphate in sediments under anoxic conditions and their implication for eutrophication control. Chem. Eng. J. 2020, 388, 124198. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Li, T. Phosphate adsorption on lanthanum loaded biochar. Chemosphere 2016, 150, 1–7. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef]
- Son, E.B.; Poo, K.M.; Chang, J.S.; Chae, K.J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.; Tsang, Y.F.; Giri, B.S.; Singh, R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616–617, 1242–1260. [Google Scholar] [CrossRef]
- Min, L.; Zhongsheng, Z.; Zhe, L.; Haitao, W. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar. Ecol. Eng. 2020, 149, 105792. [Google Scholar] [CrossRef]
- Cai, G.; Ye, Z.-L. Concentration-dependent adsorption behaviors and mechanisms for ammonium and phosphate removal by optimized Mg-impregnated biochar. J. Clean. Prod. 2022, 349, 131453. [Google Scholar] [CrossRef]
- Li, Y.; Zimmerman, A.R.; He, F.; Chen, J.; Han, L.; Chen, H.; Hu, X.; Gao, B. Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue. Sci. Total Environ. 2020, 722, 137972. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Ma, H.; Meng, Y.; Wei, T.; Wang, B. Phosphorus recovery and reuse in water bodies with simple ball-milled Ca-loaded biochar. Sci. Total Environ. 2023, 860, 160502. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, D.; Guo, H.; Lu, H.; Qiu, M.; Yang, J.; Shen, F. Solvent-Free synthesis of MgO-modified biochars for phosphorus removal from wastewater. Int. J. Environ. Res. Public Health 2022, 19, 7770. [Google Scholar] [CrossRef]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef]
- Jung, K.W.; Jeong, T.U.; Kang, H.J.; Ahn, K.H. Characteristics of biochar derived from marine macroalgae and fabrication of granular biochar by entrapment in calcium-alginate beads for phosphate removal from aqueous solution. Bioresour. Technol. 2016, 211, 108–116. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yan, W.; He, C.; Shi, Y. MgFe2O4-biochar based lanthanum alginate beads for advanced phosphate removal. Chem. Eng. J. 2020, 387, 123305. [Google Scholar] [CrossRef]
- Pinto, M.D.C.E.; da Silva, D.D.; Gomes, A.L.A.; Santos, R.; Couto, R.A.; de Novais, R.F.; Constantino, V.R.L.; Tronto, J.; Pinto, F.G. Biochar from carrot residues chemically modified with magnesium for removing phosphorus from aqueous solution. J. Clean. Prod. 2019, 222, 36–46. [Google Scholar] [CrossRef]
- Seo, J.; Yoon, H.N.; Kim, S.; Wang, Z.; Kil, T.; Lee, H.K. Characterization of reactive MgO-modified calcium sulfoaluminate cements upon carbonation. Cem. Concr. Res. 2021, 146, 106484. [Google Scholar] [CrossRef]
- Oginni, O.; Yakaboylu, G.A.; Singh, K.; Sabolsky, E.M.; Unal-Tosun, G.; Jaisi, D.; Khanal, S.; Shah, A. Phosphorus adsorption behaviors of MgO modified biochars derived from waste woody biomass resources. J. Environ. Chem. Eng. 2020, 8, 103723. [Google Scholar] [CrossRef]
- Tan, C.; Guo, Y.; Sun, J.; Li, W.; Zhang, J.; Zhao, C.; Lu, P. Structurally improved MgO adsorbents derived from magnesium oxalate precursor for enhanced CO2 capture. Fuel 2020, 278, 118379. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Liu, Z.; Tian, S.; Lu, J.; Mu, R.; Yuan, H. Coagulation removal of microplastics from wastewater by magnetic magnesium hydroxide and PAM. J. Water Process. Eng. 2021, 43, 102250. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, P.; Zhang, Z.; Liu, J.; Xiao, J.; Gao, F. Simultaneous removal of ammonia nitrogen and recovery of phosphate from swine wastewater by struvite electrochemical precipitation and recycling technology. J. Clean. Prod. 2016, 127, 302–310. [Google Scholar] [CrossRef]
- Liu, X.; Shen, F.; Qi, X. Adsorption recovery of phosphate from aqueous solution by CaO-biochar composites prepared from eggshell and rice straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Lahori, A.H.; Mahar, A. Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute. Bioresour. Technol. 2016, 215, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Kizito, S.; Luo, H.; Wu, S.; Ajmal, Z.; Lv, T.; Dong, R. Phosphate recovery from liquid fraction of anaerobic digestate using four slow pyrolyzed biochars: Dynamics of adsorption, desorption and regeneration. J. Environ. Manag. 2017, 201, 260–267. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Mckay, G.; Kochkodan, V.; Atieh, M.A.; Al-Ansari, T. A state of the art review on phosphate removal from water by biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of Rhodamine B from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Jung, K.-W.; Jeong, T.-U.; Choi, J.-W.; Ahn, K.-H.; Lee, S.-H. Adsorption of phosphate from aqueous solution using electrochemically modified biochar calcium-alginate beads: Batch and fixed-bed column performance. Bioresour. Technol. 2017, 244, 23–32. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, J.J.; Zhou, B.; Zhang, Z.; Liu, S.; Lei, S.; Xiao, R. Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. J. Clean. Prod. 2017, 147, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-J.; Jiang, H.; Tian, K.; Ding, Y.-W.; Yu, H.-Q. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ. Sci. Technol. 2013, 47, 9397–9403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, Z.; Muhmood, A.; Dong, R.; Wu, S. Probing the efficiency of magnetically modified biomass-derived biochar for effective phosphate removal. J. Environ. Manag. 2020, 253, 109730. [Google Scholar] [CrossRef] [PubMed]







| Sample | BET Surface Area (m2 g−1) | Zeta Potential (mV) |
|---|---|---|
| BC | 36.99 | −15.62 ± 0.37 |
| MgBC-450 °C-3 h-0.75 | 35.22 | 5.73 ± 0.47 |
| MgBC-450 °C-3 h-1.25 | 134.95 | 8.45 ± 0.43 |
| MgBC-450 °C-3 h-1.5 | 110.02 | 7.79 ± 0.96 |
| MgBC-450 °C-1 h-1.25 | 67.74 | 7.04 ± 0.14 |
| MgBC-450 °C -5 h-1.25 | 126.33 | 7.81 ± 0.54 |
| MgBC-550 °C-3 h-1.25 | 203.67 | 4.93 ± 0.30 |
| MgBC-650 °C-3 h-1.25 | 153.91 | 4.73 ± 0.78 |
| Kinetic Models | Parameters | FeSA-MgBC-3 |
|---|---|---|
| Experimental | qe (mg g−1) | 27.8 |
| Pseudo-first-order | qe (mg g−1) | 27.8 |
| k1 (min−1) | 0.018 | |
| R2 | 0.911 | |
| Pseudo-second-order | qe (mg g−1) | 27.8 |
| k2 (g mg−1 min−1) ×10−3 | 7.85 | |
| R2 | 0.984 |
| Isotherm Models | Parameters | FeSA-MgBC-3 |
|---|---|---|
| Langmuir | qL (mg g−1) | 53.2 |
| kL (L mg−1) | 0.292 | |
| R2 | 0.999 | |
| Freundlich | n | 5.329 |
| kF ((mg g−1) (L mg−1)1/n) | 22.86 | |
| R2 | 0.982 |
| Materials | qm for P (mg g−1) | References |
|---|---|---|
| Magnetic magnesium-modified biochar beads | 53.2 | This work |
| Lanthanum loaded biochar | 46.4 | [3] |
| FeCl3-impregnated biochar | 90.0 | [10] |
| MgFe2O4-biochar-based lanthanum alginate bead | 26.8 | [17] |
| MgO-modified biochar from waste woody | 29.2 | [20] |
| Functionalizing biochar with Mg-Al and Mg-Fe layered double hydroxides | 15.2 | [25] |
| Slow pyrolyzed biochar from corncobs | 10.9 | [27] |
| T (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | |
| FeSA-MgBC-3 | 298.15 | −12.37 | 16.96 | 98.37 |
| 303.15 | −12.86 | |||
| 313.15 | −13.84 | |||
| 323.15 | −14.83 | |||
| 333.15 | −15.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Yan, N.; Zheng, Z.; Xu, L.; Xie, H.; Chen, J. Recyclable Magnesium-Modified Biochar Beads for Efficient Removal of Phosphate from Wastewater. Nanomaterials 2023, 13, 966. https://doi.org/10.3390/nano13060966
Hu B, Yan N, Zheng Z, Xu L, Xie H, Chen J. Recyclable Magnesium-Modified Biochar Beads for Efficient Removal of Phosphate from Wastewater. Nanomaterials. 2023; 13(6):966. https://doi.org/10.3390/nano13060966
Chicago/Turabian StyleHu, Biao, Nina Yan, Zhiyu Zheng, Lei Xu, Hongde Xie, and Jingwen Chen. 2023. "Recyclable Magnesium-Modified Biochar Beads for Efficient Removal of Phosphate from Wastewater" Nanomaterials 13, no. 6: 966. https://doi.org/10.3390/nano13060966