CuInS2 Nanocrystals Embedded PMMA Composite Films: Adjustment of Polymer Molecule Weights and Application in Remote-Type White LEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CuInS2-Based NCs
2.3. Preparation of CuInS2 NCs/PMMA Composite Films
2.4. Characterizations of CuInS2 NCs/PMMA Composite Films
3. Results and Discussion
3.1. Solution Casting Method for CuInS2 NCs/PMMA Composite Films
3.2. Optical Properties of Green Emissive CuInS2 NCs/PMMA Composite Films
3.3. Optical Properties of Red Emissive CuInS2 NCs/PMMA Composite Films
3.4. Dispersibility of CuInS2 NCs in Green and Red Emissive Composite Films
3.5. Vacuum Thermal Stability of Red Emissive CuInS2 NCs/PMMA Composite Films
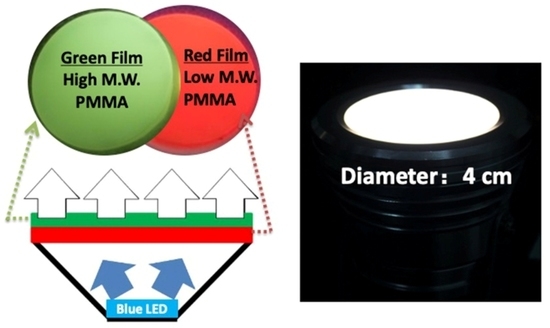
3.6. Application of CuInS2 NCs/PMMA Composite Film in Remote White LEDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovalenko, M.V.; Manna, L.; Cabot, A.; Hens, Z.; Talapin, D.V.; Kagan, C.R.; Klimov, V.I.; Rogach, A.L.; Reiss, P.; Milliron, D.J.; et al. Prospects of Nanoscience with Nanocrystals. ACS Nano 2015, 9, 1012–1057. [Google Scholar] [CrossRef]
- Pietryga, J.M.; Park, Y.S.; Lim, J.; Fidler, A.F.; Bae, W.K.; Brovelli, S.; Klimov, V.I. Spectroscopic and Device Aspects of Nanocrystal Quantum Dots. Chem. Rev. 2016, 116, 10513–10622. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of Colloidal Quantum-Dot Light-Emitting Technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Dey, A.; Ye, J.; De, A.; Debroye, E.; Ha, S.K.; Bladt, E.; Polavarapu, L. State of the Art and Prospects for Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 10775–10981. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-Processed, High-Performance Light-Emitting Diodes Based on Quantum Dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lou, Y.; Ding, S.; Zhang, W.; Wu, Z.; Yang, H.; Xu, B.; Wang, K.; Sun, X.W. Green InP/ZnSeS/ZnS Core Multi-Shelled Quantum Dots Synthesized with Aminophosphine for Effective Display Applications. Adv. Funct. Mater. 2021, 31, 2008453. [Google Scholar] [CrossRef]
- Frecker, T.; Bailey, D.; Arzeta-Ferrer, X.; McBride, J.; Rosenthal, S.J. Quantum Dots and Their Application in Lighting, Displays, and Biology. ECS J. Solid State Sci. Technol. 2015, 5, R3019. [Google Scholar] [CrossRef]
- Zhou, Q.; Bai, Z.; Lu, W.G.; Wang, Y.; Zou, B.; Zhong, H. In situ Fabrication of Halide Perovskite Nanocrystal-Embedded Polymer Composite Films with Enhanced Photoluminescence for Display Backlights. Adv. Mater. 2016, 28, 9163–9168. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Lin, X.; Qin, H.; Hu, Z.; Jin, Y.; Peng, X. Quantum Dots for Display Applications. Angew. Chem. 2020, 132, 22496–22507. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle Polymer Composites: Where Two Small Worlds Meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.G.; Ji, H.; Yan, X.; Zhong, H. Industry Outlook of Perovskite Quantum Dots for Display Applications. Nat. Nanotechnol. 2022, 17, 813–816. [Google Scholar] [CrossRef]
- Smith, M.J.; Lin, C.H.; Yu, S.; Tsukruk, V.V. Composite Structures with Emissive Quantum Dots for Light Enhancement. Adv. Opt. Mater. 2019, 7, 1801072. [Google Scholar] [CrossRef] [Green Version]
- Coe-Sullivan, S.; Liu, W.; Allen, P.; Steckel, J.S. Quantum Dots for LED Downconversion in Display Applications. ECS J. Solid State Sci. 2012, 2, R3026. [Google Scholar]
- Zhou, Q.; Bai, Z.; Lu, L.; Zhong, H. Remote Phosphor Technology for White LED Applications: Advances and Prospects. Chin. Opt. 2015, 8, 313–328. [Google Scholar] [CrossRef]
- Kim, J.K.; Luo, H.; Schubert, E.F.; Cho, J.; Sone, C.; Park, Y. Strongly Enhanced Phosphor Efficiency in GaInN White Light-Emitting Diodes Using Remote Phosphor Configuration and Diffuse Reflector Cup. Jpn. J. Appl. Phys. 2005, 44, L649. [Google Scholar] [CrossRef] [Green Version]
- Abe, S.; Joos, J.J.; Martin, L.I.; Hens, Z.; Smet, P.F. Hybrid Remote Quantum Dot/Powder Phosphor Designs for Display Backlights. Light Sci. Appl. 2017, 6, e16271. [Google Scholar] [CrossRef] [Green Version]
- Schadler, L.S.; Kumar, S.K.; Benicewicz, B.C.; Lewis, S.L.; Harton, S.E. Designed Interfaces in Polymer Nanocomposites: A Fundamental Viewpoint. MRS Bull. 2007, 32, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Green, P.F. The Structure of Chain End-Grafted Nanoparticle/Homopolymer Nanocomposites. Soft Matter 2011, 7, 7914–7926. [Google Scholar] [CrossRef]
- Li, Y.; Krentz, T.M.; Wang, L.; Benicewicz, B.C.; Schadler, L.S. Ligand Engineering of Polymer Nanocomposites: From the Simple to the Complex. ACS Appl. Mater. Inter. 2014, 6, 6005–6021. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic–Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Baek, S.; Kim, Y.; Kim, S.W. Highly Photo-Stable CsPbI3 Perovskite Quantum Dots via Thiol Ligand Exchange and Their Polymer Film Application. J. Ind. Eng. Chem. 2020, 83, 279–284. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, H.; Zhang, W.; Tan, Z.; Li, Y.; Yu, C.; Zhai, T.; Bando, Y.; Yang, S.; Zou, B. Highly Emissive and Color-Tunable CuInS2-Based Colloidal Semiconductor Nanocrystals: Off-Stoichiometry Effects and Improved Electroluminescence Performance. Adv. Funct. Mater. 2012, 22, 2081–2088. [Google Scholar] [CrossRef]
- Chen, B.; Pradhan, N.; Zhong, H. From Large-Scale Synthesis to Lighting Device Applications of ternary I–III–VI Semiconductor Nanocrystals: Inspiring Greener Material Emitters. J. Phys. Chem. Lett. 2018, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.H.; Lin, C.C.; Liu, R.S. Emission-Tunable CuInS2/ZnS Quantum Dots: Structure, Optical Properties, and Application in White Light-Emitting Diodes with High Color Rendering Index. ACS Appl. Mater. 2014, 6, 15379–15387. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.S.; Ko, M.; Jeong, S.; Shin, S.Y.; Park, S.M.; Do, Y.R.; Song, J.K. Enhancement Mechanism of Quantum Yield in Alloyed-Core/Shell Structure of ZnS–CuInS2/ZnS Quantum Dots. J. Phys. Chem. C 2021, 125, 9965–9972. [Google Scholar] [CrossRef]
- Kolny-Olesiak, J.; Weller, H. Synthesis and Application of Colloidal CuInS2 Semiconductor Nanocrystals. ACS Appl. Mater. Interfaces 2013, 5, 12221–12237. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, Q.; Li, J.; Zhang, F.; Liu, R.; Zhong, H.; Zou, B. Red Emissive CuInS2-Based Nanocrystals: A Potential Phosphor for Warm White Light-Emitting Diodes. Opt. Express 2013, 21, 10105–10110. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, H.; Wang, M.; Liu, R.; Zou, B. Integration of CuInS2-Based Nanocrystals for High Efficiency and High Colour Rendering White Light-Emitting Diodes. Nanoscale 2013, 5, 3514–3519. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly(methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Meli, L.; Arceo, A.; Green, P.F. Control of the Entropic Interactions and Phase Behavior of Athermal Nanoparticle/Homopolymer Thin Film Mixtures. Soft Matter 2009, 5, 533–537. [Google Scholar] [CrossRef]
- Hasegawa, R.; Aoki, Y.; Doi, M. Optimum Graft Density for Dispersing Particles in Polymer Melts. Macromolecules 1996, 29, 6656–6662. [Google Scholar] [CrossRef]
- Matsen, M.W.; Gardiner, J.M. Autophobic Dewetting of Homopolymer on a Brush and Entropic Attraction Between Opposing Brushes in a Homopolymer Matrix. J. Chem. Phys. 2001, 115, 2794–2804. [Google Scholar] [CrossRef]
- Dutta, N.; Green, D. Nanoparticle Stability in Semidilute and Concentrated Polymer Solutions. Langmuir 2008, 24, 5260–5269. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Yu, Y.; Zuo, S. Preparation and Multicolored Fluorescent Properties of CdTe Quantum Dots/Polymethylmethacrylate Composite Films. J. Alloys Compd. 2015, 647, 578–584. [Google Scholar] [CrossRef]
- Doblas, D.; Kister, T.; Cano-Bonilla, M.; González-García, L.; Kraus, T. Colloidal Solubility and Agglomeration of Apolar Nanoparticles in Different Solvents. Nano Lett. 2019, 19, 5246–5252. [Google Scholar] [CrossRef] [PubMed]
- Akdas, T.; DAistaso, M.; Kuhri, S.; Winter, B.; Birajdar, B.; Spiecker, E.; Peukert, W. The Effects of Post-Processing on the Surface and the Optical Properties of Copper Indium Sulfide Quantum Dots. J. Colloid Interface Sci. 2015, 445, 337–347. [Google Scholar] [CrossRef]
- Li, Y.; Tao, P.; Viswanath, A.; Benicewicz, B.C.; Schadler, L.S. Bimodal Surface Ligand Engineering: The Key to Tunable Nanocomposites. Langmuir 2013, 29, 1211–1220. [Google Scholar] [CrossRef]
- Luo, H.; Kim, J.K.; Schubert, E.F.; Cho, J.; Sone, C.; Park, Y. Analysis of High-Power Packages for Phosphor-Based White-Light-Emitting Diodes. Appl. Phys. Lett. 2005, 86, 243505. [Google Scholar] [CrossRef] [Green Version]







| M.W. of PMMA | T% | T% @ 30 h | WL | WL @ 30 h | Red-Shift |
|---|---|---|---|---|---|
| 15 k | 76.25 | 42.01 | 658.8 nm | 666.4 nm | 7.6 nm |
| 550 k/15 k 1:4 | 75.68 | 44.69 | 661.4 nm | 666.6 nm | 5.2 nm |
| 550 k/15 k 1:1 | 73.58 | 51.12 | 661.2 nm | 669.4 nm | 8.2 nm |
| 550 k/15 k 4:1 | 73.52 | 48.66 | 659.2 nm | 668.8 nm | 9.6 nm |
| 550 k | 68.67 | 50.53 | 662.4 nm | 667.2 nm | 4.8 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Shang, Z. CuInS2 Nanocrystals Embedded PMMA Composite Films: Adjustment of Polymer Molecule Weights and Application in Remote-Type White LEDs. Nanomaterials 2023, 13, 1085. https://doi.org/10.3390/nano13061085
Zhou Q, Shang Z. CuInS2 Nanocrystals Embedded PMMA Composite Films: Adjustment of Polymer Molecule Weights and Application in Remote-Type White LEDs. Nanomaterials. 2023; 13(6):1085. https://doi.org/10.3390/nano13061085
Chicago/Turabian StyleZhou, Qingchao, and Zhongyi Shang. 2023. "CuInS2 Nanocrystals Embedded PMMA Composite Films: Adjustment of Polymer Molecule Weights and Application in Remote-Type White LEDs" Nanomaterials 13, no. 6: 1085. https://doi.org/10.3390/nano13061085