Synthesis and Characterization of Porous MgO Nanosheet-Modified Activated Carbon Fiber Felt for Fluoride Adsorption
Abstract
:1. Introduction
2. Materials and Methods
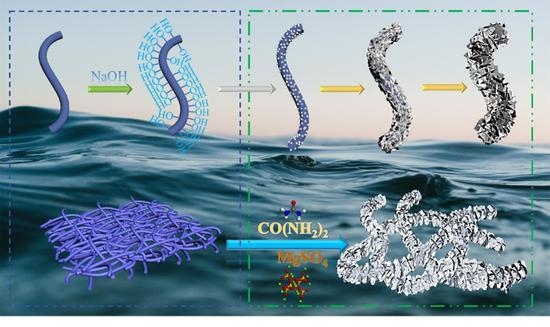
2.1. Preparation
2.2. Characterization of the MgO@ACFF
2.3. Adsorption Experiments
3. Results and Discussion
3.1. Characterization of Materials
3.2. Adsorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhatnagar, A.; Kumar, E.; Sillanpää, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Pang, T.; Chan, T.S.A.; Jande, Y.A.C.; Shen, J. Removal of fluoride from water using activated carbon fibres modified with zirconium by a drop-coating method. Chemosphere 2020, 255, 126950. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019, 173, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xia, H.; Zhang, Q.; Jiang, G.; Cai, W.; Hu, W. Adsorption of cadmium(II) in wastewater by magnesium oxide modified biochar. Arab. J. Chem. 2022, 15, 104059. [Google Scholar] [CrossRef]
- Jeirani, Z.; Niu, C.H.; Soltan, J. Adsorption of emerging pollutants on activated carbon. Rev. Chem. Eng. 2016, 33, 491–522. [Google Scholar] [CrossRef]
- Qi, D.; Lv, F.; Wei, T.; Jin, M.; Meng, G.; Zhang, S.; Liu, Q.; Liu, W.; Ma, D.; Hamdy, M.S.; et al. High-efficiency electrocatalytic NO reduction to NH 3 by nanoporous VN. Nano Res. Energy 2022, 1, e9120022. [Google Scholar] [CrossRef]
- Abaalkhail, A.A.; Alshammari, B.A.; Almutairi, G.N.; Alenazey, F.S.; Alotibi, M.F.; Alenad, A.M.; Alharbi, A.G.; Almoneef, T.S.; AlOtaibi, B.M. Enhancing the Performance of a Metal-Free Self-Supported Carbon Felt-Based Supercapacitor with Facile Two-Step Electrochemical Activation. Nanomaterials 2022, 12, 427. [Google Scholar] [CrossRef]
- Tefera, N.; Mulualem, Y.; Fito, J. Adsorption of Fluoride from Aqueous Solution and Groundwater onto Activated Carbon of Avocado Seeds. Water Conserv. Sci. Eng. 2020, 5, 187–197. [Google Scholar] [CrossRef]
- Vences-Alvarez, E.; Velazquez-Jimenez, L.H.; Chazaro-Ruiz, L.F.; Díaz-Flores, P.E.; Rangel-Mendez, J.R. Fluoride removal in water by a hybrid adsorbent lanthanum–carbon. J. Colloid Interfaces Sci. 2015, 455, 194–202. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, X.; Panther, J.G.; Wang, Q.; Chakir, S.; Ding, Y.; Huang, Y.; Wang, H. Micro/nanostructured MgO hollow spheres with selective adsorption performance and their application for fluoride monitoring in water. Sep. Purif. Technol. 2022, 299, 121703. [Google Scholar] [CrossRef]
- Jin, Z.; Jia, Y.; Zhang, K.-S.; Kong, L.-T.; Sun, B.; Shen, W.; Meng, F.-L.; Liu, J.-H. Effective removal of fluoride by porous MgO nanoplates and its adsorption mechanism. J. Alloy. Compd. 2016, 675, 292–300. [Google Scholar] [CrossRef]
- Shahkarami, S.; Dalai, A.K.; Soltan, J. Enhanced CO2 Adsorption Using MgO-Impregnated Activated Carbon: Impact of Preparation Techniques. Ind. Eng. Chem. Res. 2016, 55, 5955–5964. [Google Scholar] [CrossRef]
- Siriwardane, I.W.; Udangawa, R.; de Silva, R.M.; Kumarasinghe, A.; Acres, R.G.; Hettiarachchi, A.; Amaratunga, G.A.; de Silva, K.N. Synthesis and characterization of nano magnesium oxide impregnated granular activated carbon composite for H2S removal applications. Mater. Des. 2017, 136, 127–136. [Google Scholar] [CrossRef]
- Ghaemi, A.; Mashhadimoslem, H.; Izadpanah, P.Z. NiO and MgO/activated carbon as an efficient CO2 adsorbent: Characterization, modeling, and optimization. Int. J. Environ. Sci. Technol. 2021, 19, 727–746. [Google Scholar] [CrossRef]
- Yue, X.; Zhao, J.; Shi, H.; Chi, Y.; Salam, M. Preparation of composite adsorbents of activated carbon supported MgO/MnO2 and adsorption of Rhodamine B. Water Sci. Technol. 2020, 81, 906–914. [Google Scholar] [CrossRef] [Green Version]
- Gueorguiev, G.; Stafström, S.; Hultman, L. Nano-wire formation by self-assembly of silicon–metal cage-like molecules. Chem. Phys. Lett. 2008, 458, 170–174. [Google Scholar] [CrossRef]
- dos Santos, R.B.; Rivelino, R.; Mota, F.D.B.; Kakanakova-Georgieva, A.; Gueorguiev, G.K. Feasibility of novel (H3C)nX(SiH3)3−ncompounds (X = B, Al, Ga, In): Structure, stability, reactivity, and Raman characterization from ab initio calculations. Dalton Trans. 2015, 44, 3356–3366. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Hong, M.W.; Yoon, H.C.; Yi, K.B. Effects of MgCl2 loading on ammonia capacity of activated carbon for application in temperature swing adsorption, pressure swing adsorption, and pressure-temperature swing adsorption process. Korean J. Chem. Eng. 2022, 39, 2775–2782. [Google Scholar] [CrossRef]
- Mullick, A.; Neogi, S. Ultrasound assisted synthesis of Mg-Mn-Zr impregnated activated carbon for effective fluoride adsorption from water. Ultrason. Sonochem. 2018, 50, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Propolsky, D.; Romanovskaia, E.; Kwapinski, W.; Romanovski, V. Modified activated carbon for deironing of underground water. Environ. Res. 2019, 182, 108996. [Google Scholar] [CrossRef]
- Nazarian, R.; Desch, R.J.; Thiel, S.W. Kinetics and equilibrium adsorption of phosphate on lanthanum oxide supported on activated carbon. Colloids Surfaces A: Physicochem. Eng. Asp. 2021, 624, 126813. [Google Scholar] [CrossRef]
- Ma, J.; Li, F.; Qian, T.; Liu, H.; Liu, W.; Zhao, D. Natural organic matter resistant powder activated charcoal supported titanate nanotubes for adsorption of Pb(II). Chem. Eng. J. 2017, 315, 191–200. [Google Scholar] [CrossRef]
- Yaseen, M.; Ullah, S.; Ahmad, W.; Subhan, S.; Subhan, F. Fabrication of Zn and Mn loaded activated carbon derived from corn cobs for the adsorptive desulfurization of model and real fuel oils. Fuel 2020, 284, 119102. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Li, D.; Liu, Z.-Q.; Tao, Q.; Zhu, Y.; Yang, J.; Zhang, Y.-M. Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La(OH)3 -modified exfoliated vermiculites as highly efficient phosphate adsorbents. Chem. Eng. J. 2014, 236, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.-X.; Qu, J.-H.; Liu, R.-P.; Lan, H.-C. Adsorption of fluoride onto different types of aluminas. Chem. Eng. J. 2012, 189, 126–133. [Google Scholar] [CrossRef]
- Liu, R.; Gong, W.; Lan, H.; Gao, Y.; Liu, H.; Qu, J. Defluoridation by freshly prepared aluminum hydroxides. Chem. Eng. J. 2011, 175, 144–149. [Google Scholar] [CrossRef]
- Nie, Y.; Hu, C.; Kong, C. Enhanced fluoride adsorption using Al (III) modified calcium hydroxyapatite. J. Hazard. Mater. 2012, 233, 194–199. [Google Scholar] [CrossRef]
- Mullick, A.; Neogi, S. Acoustic cavitation induced synthesis of zirconium impregnated activated carbon for effective fluoride scavenging from water by adsorption. Ultrason. Sonochem. 2018, 45, 65–77. [Google Scholar] [CrossRef]
- Wu, P.; Wu, J.; Xia, L.; Liu, Y.; Xu, L.; Song, S. Adsorption of fluoride at the interface of water with calcined magnesium-ferri-lanthanum hydrotalcite-like compound. RSC Adv. 2017, 7, 26104–26112. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-X.; Xu, D.; Li, X.-Q.; Liu, W.-C.; Jia, Y. Excellent fluoride removal properties of porous hollow MgO microspheres. New J. Chem. 2014, 38, 5445–5452. [Google Scholar] [CrossRef]
- Moriyama, S.; Sasaki, K.; Hirajima, T. Effect of calcination temperature on Mg–Al bimetallic oxides as sorbents for the removal of F− in aqueous solutions. Chemosphere 2014, 95, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, C.S.; Viswanathan, N.; Meenakshi, S. Defluoridation of water using magnesia/chitosan composite. J. Hazard. Mater. 2009, 163, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, W.; Yang, H.; Ye, B.-C. Efficient removal of fluoride from aqueous solutions using 3D flower-like hierarchical zinc-magnesium-aluminum ternary oxide microspheres. Chem. Eng. J. 2019, 380, 122459. [Google Scholar] [CrossRef]
- Borgohain, X.; Boruah, A.; Sarma, G.K.; Rashid, H. Rapid and extremely high adsorption performance of porous MgO nanostructures for fluoride removal from water. J. Mol. Liq. 2020, 305, 112799. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Ni, Y.; Liu, Z.; Chen, J.; Liang, X. Temperature- and pH-Dependent Morphology and FT−IR Analysis of Magnesium Carbonate Hydrates. J. Phys. Chem. B 2006, 110, 12969–12973. [Google Scholar] [CrossRef]
- Nefedov, V.; Buslaev, Y.; Sergushin, N.; Kokunov, Y.; Kovalev, V.; Bayer, L. Electronic structure patterns of isoelectronic compounds. J. Electron Spectrosc. Relat. Phenom. 1975, 6, 221–229. [Google Scholar] [CrossRef]
- Foerch, R.; Beamson, G.; Briggs, D. XPS valence band analysis of plasma-treated polymers. Surf. Interface Anal. 1991, 17, 842–846. [Google Scholar] [CrossRef]
- Uhl, F.; Staemmler, V. An ab initio study of the O1s and Mg1s, Mg2s, Mg2p core electron binding energies in bulk MgO. J. Electron Spectrosc. Relat. Phenom. 2019, 233, 90–96. [Google Scholar] [CrossRef]
- Sosulnikov, M.; Teterin, Y. X-ray photoelectron studies of Ca, Sr and Ba and their oxides and carbonates. J. Electron Spectrosc. Relat. Phenom. 1992, 59, 111–126. [Google Scholar] [CrossRef]
- Haycock, D.E.; Kasrai, M.; Nicholls, C.J.; Urch, D.S. The electronic structure of magnesium hydroxide (brucite) using X-ray emission, X-ray photoelectron, and auger spectroscopy. J. Chem. Soc. Dalton Trans. 1978, 12, 1791–1796. [Google Scholar] [CrossRef]















| Material | Loading Amount (Weight%) |
|---|---|
| AC-Mg [18] | 22.58 |
| AC-Mg [19] | 0.65 |
| AC-Mn [19] | 3.56 |
| AC-Fe-U [20] | 17.33 ± 0.71 |
| AC-La [21] | 12 |
| PAC-TNTs [22] | 70.94 |
| AC-Zn/AC-Mn [23] | 16.76/14.44 |
| EV-La(OH)3 [24] | 31.52 |
| This work | 810 |
| Equations | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Viscosity | qm (mg/g) | KL (L/mg) | R2 | KF (mg/g) | 1/n | R2 |
| 0.1 M | 86.356 | 0.040 | 0.989 | 6.970 | 2.144 | 0.823 |
| 0.5 M | 136.799 | 0.018 | 0.961 | 8.321 | 2.196 | 0.998 |
| 1 M | 253.165 | 0.009 | 0.928 | 6.230 | 1.689 | 0.991 |
| 2 M | 150.60 | 0.023 | 0.991 | 8.215 | 1.952 | 0.888 |
| Commercial MgO | 45.186 | 0.002 | 0.636 | 0.408 | 1.242 | 0.986 |
| Adsorbent | Adsorption Capacity (mg/g) | Dose (g/L) | pH |
|---|---|---|---|
| Alumina [25] | 83.3 | 1 | 6 |
| Aluminum hydroxide [26] | 110 | 0.081 | 6 |
| Al-modified hydroxyapatite [27] | 32.57 | 5 | 7 |
| La (III)–modified GAC [9] | 9.96 | 0.1 | 7 |
| AC-Zr [28] | 5.4 | 2 | 5.44 |
| AC-Mn-Mg-Zr [19] | 26.67 | 1 | 11.9 |
| Mg-Fe-La hydrotalcite-like compound [29] | 59.98 | 0.5 | 6.8 |
| orous hollow MgO microspheres [30] | >120 | 1 | 7 |
| Mg–Al bimetallic oxides [31] | 89.3 | 1 | 6 |
| MgO/chitosan [32] | >4.44 | 2 | 7 |
| porous MgO nanosheets [11] | >185.5 | 1 | 7 |
| MgO@ACFF (This work) | >212.2 | 0.3 | neutral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.-C.; Xu, M.-D.; Jin, Z.; Xiao, Y.-F.; Chao, Y.; Li, J.; Chen, S.-H.; Ding, Y. Synthesis and Characterization of Porous MgO Nanosheet-Modified Activated Carbon Fiber Felt for Fluoride Adsorption. Nanomaterials 2023, 13, 1082. https://doi.org/10.3390/nano13061082
Wang D-C, Xu M-D, Jin Z, Xiao Y-F, Chao Y, Li J, Chen S-H, Ding Y. Synthesis and Characterization of Porous MgO Nanosheet-Modified Activated Carbon Fiber Felt for Fluoride Adsorption. Nanomaterials. 2023; 13(6):1082. https://doi.org/10.3390/nano13061082
Chicago/Turabian StyleWang, De-Cai, Min-Da Xu, Zhen Jin, Yi-Fan Xiao, Yang Chao, Jie Li, Shao-Hua Chen, and Yi Ding. 2023. "Synthesis and Characterization of Porous MgO Nanosheet-Modified Activated Carbon Fiber Felt for Fluoride Adsorption" Nanomaterials 13, no. 6: 1082. https://doi.org/10.3390/nano13061082