Preparation of Functional Nanoparticles-Loaded Magnetic Carbon Nanohorn Nanocomposites towards Composite Treatment
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instruments
2.3. Preparation of Materials
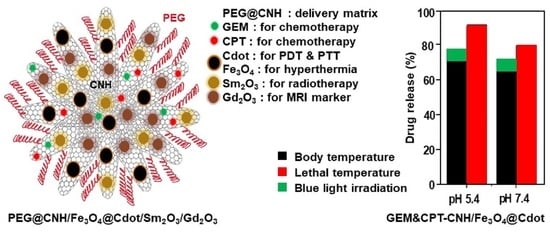
2.4. Drug Loading and Release
2.5. Viability Test
3. Results and Discussion
3.1. Characterization of Materials
3.2. Drug Loading and Release of DOX and GEM
3.3. Co-Loading and Release of Drugs
3.4. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ACS. Cancer Facts & Figures 2019; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- VanDyke, D.; Kyriacopulos, P.; Yassini, B.; Wright, A.; Burkhart, E.; Jacek, S.; Pratt, M.; Peterson, C.; Rai, P. Nanoparticle based combination treatments for targeting multiple hallmarks of cancer. Int. J. Nano Stud. Technol. 2016, 1, 1–18. [Google Scholar] [CrossRef]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional Phototheranostic Nanomedicine for Cancer Imaging and Treatment. Mater. Today Bio 2019, 5, 100035. [Google Scholar] [CrossRef] [PubMed]
- Kalash, R.S.; Lakshmanan, V.K.; Cho, C.S.; Park, I.K. Theranostics. In Biomaterials Nanoarchitectonics, 1st ed.; Ebara, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 197–215. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized hydrophilic superparamagnetic iron oxide nanoparticles for magnetic fluid hyperthermia application in liver cancer treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hosseinkhani, H.; Hosseinkhani, M.; Boutry, S.; Simchi, A.; Journeay, W.S.; Subramani, K.; Laurent, S. Magnetic resonance imaging tracking of stem cells in vivo using iron oxide nanoparticles as a tool for the advancement of clinical regenerative medicine. Chem. Rev. 2011, 111, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Ahmad, T.; Rhee, I.; Chang, Y.; Jin, S.-U.; Hong, S. Carbon-coated iron oxide nanoparticles as contrast agents in magnetic resonance imaging. Nanoscale Res. Lett. 2012, 7, 1–5. [Google Scholar] [CrossRef]
- Teng, X.; Li, F.; Lu, C.; Li, B. Carbon dot-assisted luminescence of singlet oxygen: The generation dynamics but not the cumulative amount of singlet oxygen is responsible for the photodynamic therapy efficacy. Nanoscale Horiz. 2020, 5, 978–985. [Google Scholar] [CrossRef]
- Do, T.T.A.; Imae, T. Photodynamic and Photothermal Effects of Carbon Dots-Coated Magnetite- and Porphyrin-Conjugated Confeito-Like Gold Nanoparticles. Bull. Chem. Soc. Jpn. 2021, 94, 2079–2088. [Google Scholar] [CrossRef]
- Hu, Z.; Ahrén, M.; Selegård, L.; Skoglund, C.; Söderlind, F.; Engström, M.; Zhang, X.; Uvdal, K. Highly Water-Dispersible Surface-Modified Gd2O3 Nanoparticles for Potential Dual-Modal Bioimaging. Chem. Eur. J. 2013, 19, 12658–12667. [Google Scholar] [CrossRef]
- Di Corato, R.; Gazeau, F.; Le Visage, C.; Fayol, D.; Levitz, P.; Lux, F.; Letourneur, D.; Luciani, N.; Tillement, O.; Wilhelm, C. High-resolution cellular MRI: Gadolinium and iron oxide nanoparticles for in-depth dual-cell imaging of engineered tissue constructs. ACS Nano 2013, 7, 7500–7512. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Rathke, H.; Fink, R.; Dendl, K.; Debus, J.; Mier, W.; Jager, D.; Lindner, T.; Haberkorn, U. [(153)Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3011–3013. [Google Scholar] [CrossRef]
- Murray, I.; Du, Y. Systemic Radiotherapy of Bone Metastases With Radionuclides. Clin. Oncol. 2021, 33, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Parlak, Y.; Gumuser, G.; Sayit, E. Samarium-153 Therapy and Radiation Dose for Prostate Cancer. In Prostate Cancer: Leading-edge Diagnostic Procedures and Treatments; Mohan, R., Ed.; InTech: London, UK, 2016; pp. 81–89. [Google Scholar] [CrossRef]
- Anderson, P.; Nuñez, R. Samarium lexidronam (153Sm-EDTMP): Skeletal radiation for osteoblastic bone metastases and osteosarcoma. Expert Rev. Anticancer Ther. 2007, 7, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Yeong, C.H.; Wong, Y.H.; McKenzie, M.; Kasbollah, A.; Md Shah, M.N.; Perkins, A.C. Neutron-activated theranostic radionuclides for nuclear medicine. Nucl. Med. Biol. 2020, 90–91, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Cirillo, G.; Saletta, F.; Michniewicz, F.; Nicoletta, F.P.; Vittorio, O.; Hampel, S.; Iemma, F. Carbon Nanohorns as Effective Nanotherapeutics in Cancer Therapy. C 2020, 7, 3. [Google Scholar] [CrossRef]
- Ajima, K.; Murakami, T.; Mizoguchi, Y.; Tsuchida, K.; Ichihashi, T.; Iijima, S.; Yudasaka, M. Enhancement of in vivo anticancer effects of cisplatin by incorporation inside single-wall carbon nanohorns. ACS Nano 2008, 2, 2057–2064. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, B.-P.; Liang, J.; Wen, C.; Shen, X.-C. Phycocyanin functionalized single-walled carbon nanohorns hybrid for near-infrared light-mediated cancer phototheranostics. Carbon 2019, 143, 814–827. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Zhang, F.; Yin, Y.; Mei, L.; Song, F.; Tao, M.; Yue, W.; Zhong, W. Overcoming Multidrug Resistance by A Combination of Chemotherapy and Photothermal Therapy Mediated by Carbon Nanohorns. J. Mater. Chem. B 2016, 4, 6043–6051. [Google Scholar] [CrossRef]
- Lage, T.; Rodrigues, R.O.; Catarino, S.; Gallo, J.; Bañobre-López, M.; Minas, G. Graphene-based magnetic nanoparticles for theranostics: An overview for their potential in clinical application. Nanomaterials 2021, 11, 1073. [Google Scholar] [CrossRef]
- Liu, X.; Yan, B.; Li, Y.; Ma, X.; Jiao, W.; Shi, K.; Zhang, T.; Chen, S.; He, Y.; Liang, X.-J. Graphene oxide-grafted magnetic nanorings mediated magnetothermodynamic therapy favoring reactive oxygen species-related immune response for enhanced antitumor efficacy. ACS Nano 2020, 14, 1936–1950. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, J.; Hao, Y.; Zhang, P.; Zhao, Y.; Meng, D.; Li, D.; Chang, J.; Zhang, Z. Magnetic multi-walled carbon nanotubes for tumor theranostics. J. Biomed. Nanotechnol. 2015, 11, 1653–1661. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Liang, J.; Li, S.; Hu, L.F.; Liang, H.; Kuo, W.S.; Shen, X.C. Smart Phototheranostics based on Carbon Nanohorns for Precise Imaging-Guided Post-PDT toward Residual Tumor Cells after Initial Phototherapy. Chem. Eur. J. 2022, 29, e202203196. [Google Scholar] [CrossRef] [PubMed]
- Isaac, K.M.; Sabaraya, I.V.; Ghousifam, N.; Das, D.; Pekkanen, A.M.; Romanovicz, D.K.; Long, T.E.; Saleh, N.B.; Rylander, M.N. Functionalization of single-walled carbon nanohorns for simultaneous fluorescence imaging and cisplatin delivery in vitro. Carbon 2018, 138, 309–318. [Google Scholar] [CrossRef]
- Dinan, N.M.; Atyabi, F.; Rouini, M.-R.; Amini, M.; Golabchifar, A.-A.; Dinarvand, R. Doxorubicin loaded folate-targeted carbon nanotubes: Preparation, cellular internalization, in vitro cytotoxicity and disposition kinetic study in the isolated perfused rat liver. Mater. Sci. Eng. C 2014, 39, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Fan, J.; Yudasaka, M.; Iijima, S.; Shiba, K. Solubilization of Single-Wall Carbon Nanohorns Using a PEG− Doxorubicin Conjugate. Mol. Pharm. 2006, 3, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Ton, K.A.; Syu, Y.-W.; Xu, J.-J.; Imae, T. Preparation of Sm, Gd and Fe Oxide Nanoparticle-Polydopamine Multicomponent Nanocomposites. Bull. Chem. Soc. Jpn. 2019, 92, 1280–1288. [Google Scholar] [CrossRef]
- Efa, M.T.; Imae, T. Hybridization of carbon-dots with ZnO nanoparticles of different sizes. J. Taiwan Inst. Chem. Eng. 2018, 92, 112–117. [Google Scholar] [CrossRef]
- Su, C.-H.; Soendoro, A.; Okayama, S.; Rahmania, F.J.; Nagai, T.; Imae, T.; Tsutsumiuchi, K.; Kawai, N. Drug release stimulated by magnet and light on magnetite-and carbon dot-loaded carbon nanohorn. Bull. Chem. Soc. Jpn. 2022, 95, 582–594. [Google Scholar] [CrossRef]
- Fite, M.C.; Rao, J.-Y.; Imae, T. Effect of External Magnetic Field on Hybrid Supercapacitors of Nitrogen-Doped Graphene with Magnetic Metal Oxides. Bull. Chem. Soc. Jpn. 2020, 93, 1139–1149. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Hsieh, H.-L.; Viswanathan, G.; Voon, S.H.; Kue, C.S.; Saw, W.S.; Yeong, C.H.; Azlan, C.A.; Imae, T.; Kiew, L.V.; et al. Multifunctional carbon-coated magnetic sensing graphene oxide-cyclodextrin nanohybrid for potential cancer theranosis. J. Nanopart. Res. 2017, 19, 359. [Google Scholar] [CrossRef]
- Siriviriyanun, A.; Popova, M.; Imae, T.; Kiew, L.V.; Looi, C.Y.; Wong, W.F.; Lee, H.B.; Chung, L.Y. Preparation of graphene oxide/dendrimer hybrid carriers for delivery of doxorubicin. Chem. Eng. J. 2015, 281, 771–781. [Google Scholar] [CrossRef]
- Siriviriyanun, A.; Imae, T.; Calderó, G.; Solans, C. Phototherapeutic functionality of biocompatible graphene oxide/dendrimer hybrids. Colloids Surf. B 2014, 121, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.A.; Grijalvo, S.; Imae, T.; Garcia-Celma, M.J.; Rodríguez-Abreu, C. A nanocellulose-based platform towards targeted chemo-photodynamic/photothermal cancer therapy. Carbohydr. Polym. 2021, 270, 118366. [Google Scholar] [CrossRef] [PubMed]
- Siriviriyanun, A.; Tsai, Y.-J.; Voon, S.H.; Kiew, S.F.; Imae, T.; Kiew, L.V.; Looi, C.Y.; Wong, W.F.; Lee, H.B.; Chung, L.Y. Cyclodextrin-and dendrimer-conjugated graphene oxide as a nanocarrier for the delivery of selected chemotherapeutic and photosensitizing agents. Mater. Sci. Eng. C 2018, 89, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Krathumkhet, N.; Sabrina; Imae, T.; Krafft, M.P. Nitric Oxide Gas in Carbon Nanohorn/Fluorinated Dendrimer/Fluorinated Poly(ethylene glycol)-Based Hierarchical Nanocomposites as Therapeutic Nanocarriers. ACS Appl. Bio Mater. 2021, 4, 2591–2600. [Google Scholar] [CrossRef]
- Bazzi, R.; Flores, M.; Louis, C.; Lebbou, K.; Zhang, W.; Dujardin, C.; Roux, S.; Mercier, B.; Ledoux, G.; Bernstein, E. Synthesis and properties of europium-based phosphors on the nanometer scale: Eu2O3, Gd2O3: Eu, and Y2O3: Eu. J. Colloid Interface Sci. 2004, 273, 191–197. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.; Yang, W.; Tian, J.; Guan, F.; Ma, Y.; Hou, J.; Kang, J.; Wang, Y. Preparation of samarium oxide nanoparticles and its catalytic activity on the esterification. Mater. Chem. Phys. 2003, 77, 65–69. [Google Scholar] [CrossRef]
- Pramoda, K.; Moses, K.; Ikram, M.; Vasu, K.; Govindaraj, A.; Rao, C.N.R. Synthesis, Characterization and Properties of Single-Walled Carbon Nanohorns. J. Clust. Sci. 2013, 25, 173–188. [Google Scholar] [CrossRef]
- Selvamani, V. Stability Studies on Nanomaterials Used in Drugs. In Characterization and Biology of Nanomaterials for Drug Delivery, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–444. [Google Scholar] [CrossRef]
- Zhu, S.; Panne, U.; Rurack, K. A rapid method for the assessment of the surface group density of carboxylic acid-functionalized polystyrene microparticles. Analyst 2013, 138, 2924–2930. [Google Scholar] [CrossRef]
- Salas, G.; Veintemillas-Verdaguer, S.; Morales, M.d.P. Relationship between physico-chemical properties of magnetic fluids and their heating capacity. Int. J. Hyperth. 2013, 29, 768–776. [Google Scholar] [CrossRef]
- Vovusha, H.; Banerjee, D.; Yadav, M.K.; Perrozzi, F.; Ottaviano, L.; Sanyal, S.; Sanyal, B. Binding characteristics of anticancer drug doxorubicin with two-dimensional graphene and graphene oxide: Insights from density functional theory calculations and fluorescence spectroscopy. J. Phys. Chem. C 2018, 122, 21031–21038. [Google Scholar] [CrossRef]
- Wattanakul, K.; Imae, T.; Chang, W.-W.; Chu, C.-C.; Nakahata, R.; Yusa, S.-I. Oligopeptide-side chained alginate nanocarrier for melittin-targeted chemotherapy. Polym. J. 2019, 51, 771–780. [Google Scholar] [CrossRef]
- Han, U.; Seo, Y.; Hong, J. Effect of pH on the structure and drug release profiles of layer-by-layer assembled films containing polyelectrolyte, micelles, and graphene oxide. Sci. Rep. 2016, 6, 24158. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Peng, S.; Tsai, H.-C. Drug carrier for photodynamic cancer therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xie, J.; Branford-White, C.; Quan, J.; Zhu, L. In vitro controlled release of polymeric drug-saccharide conjugates with ketoprofen, ibuprofen, and naproxen pendants. J. Appl. Polym. Sci. 2011, 121, 1654–1660. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb. Protoc. 2016, 2016, pdb-rot087379. [Google Scholar] [CrossRef]










| Sample | Average Hydrodynamic Diameter (nm) | Zeta Potential (mV) |
|---|---|---|
| CNH | 117.7 ± 26.3 | −41.7 ± 4.9 |
| CNH/Sm2O3 | 127.0 ± 11.4 | −15.4 ± 0.9 |
| CNH/Gd2O3 | 131.3 ± 18.1 | −25.9 ± 2.2 |
| CNH/Fe3O4 | 132.1 ± 11.9 | −30.2 ± 1.4 |
| CNH/Fe3O4@Cdot | 135.7 ± 12.3 | −33.7 ± 1.0 |
| CNH/Fe3O4/Sm2O3/Gd2O3 | 188.0 ± 32.5 | −28.8 ± 1.2 |
| CNH/Fe3O4@Cdot/Sm2O3/Gd2O3 | 199.8 ± 12.3 | −32.1 ± 1.9 |
| PEG@CNH/Fe3O4/Sm2O3/Gd2O3 | 201.7 ± 6.7 | −27.6 ± 2.8 |
| PEG@CNH/Fe3O4@Cdot/Sm2O3/Gd2O3 | 207.0 ± 13.9 | −30.8 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmania, F.J.; Huang, Y.-S.; Workie, Y.A.; Imae, T.; Kondo, A.; Miki, Y.; Imai, R.; Nagai, T.; Nakagawa, H.; Kawai, N.; et al. Preparation of Functional Nanoparticles-Loaded Magnetic Carbon Nanohorn Nanocomposites towards Composite Treatment. Nanomaterials 2023, 13, 839. https://doi.org/10.3390/nano13050839
Rahmania FJ, Huang Y-S, Workie YA, Imae T, Kondo A, Miki Y, Imai R, Nagai T, Nakagawa H, Kawai N, et al. Preparation of Functional Nanoparticles-Loaded Magnetic Carbon Nanohorn Nanocomposites towards Composite Treatment. Nanomaterials. 2023; 13(5):839. https://doi.org/10.3390/nano13050839
Chicago/Turabian StyleRahmania, Fitriani Jati, Yi-Shou Huang, Yitayal Admassu Workie, Toyoko Imae, Anna Kondo, Yukiko Miki, Ritsuko Imai, Takashi Nagai, Hiroshi Nakagawa, Noriyasu Kawai, and et al. 2023. "Preparation of Functional Nanoparticles-Loaded Magnetic Carbon Nanohorn Nanocomposites towards Composite Treatment" Nanomaterials 13, no. 5: 839. https://doi.org/10.3390/nano13050839