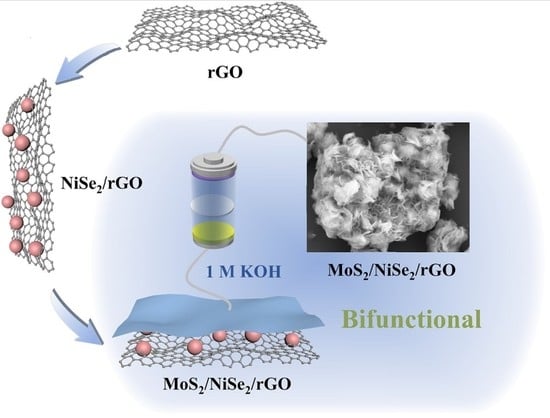
MoS2/NiSe2/rGO Multiple-Interfaced Sandwich-like Nanostructures as Efficient Electrocatalysts for Overall Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NiSe2/rGO
2.3. Preparation of MoS2/NiSe2/rGO
2.4. Morphological and Structural Characterization
2.5. Electrocatalysis Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kittner, N.; Lill, F.; Kammen, D. Energy storage deployment and innovation for the clean energy transition. Nat. Energy 2017, 2, 17125. [Google Scholar] [CrossRef] [Green Version]
- Wang, R. The dynamics of the peel. Nat. Catal. 2020, 3, 333–334. [Google Scholar] [CrossRef]
- Xia, T.; Zhao, K.; Zhu, Y.; Bai, X.; Gao, H.; Wang, Z.; Gong, Y.; Feng, M.; Li, S.; Zheng, Q.; et al. Mixed-dimensional Pt-Ni alloy polyhedral nanochains as bifunctional electrocatalysts for direct methanol fuel cell. Adv. Mater. 2022, 35, 2206508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xia, T.; Huang, H.; Liu, J.; Zhu, M.; Yu, H.; Xu, W.; Huo, Y.; He, C.; Shen, S.; et al. Autocatalytic reduction-assisted synthesis of segmented porous PtTe nanochains for enhancing methanol oxidation reaction. Nano Res. Energy 2023, 2, e9120041. [Google Scholar] [CrossRef]
- Seh, Z.; Kibsgaard, J.; Dickens, C.; Chorkendorff, I.; Norskov, J.; Jaramillo, T. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, aad4998. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Li, A.; Wang, C.; Zhou, W.; Liu, S.; Guo, L. Interfacial electron transfer of Ni2P–NiP2 polymorphs inducing enhanced electrochemical properties. Adv. Mater. 2018, 30, 1803590. [Google Scholar] [CrossRef]
- Wilhelm, M.; Bastos, A.; Neves, C.; Martins, R.; Tedim, J. Ni-Fe layered double hydroxides for oxygen evolution reaction: Impact of Ni/Fe ratio and crystallinity. Mater. Des. 2021, 212, 110188. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, X.; Wang, H.; Liu, G.; Wang, G.; Zhang, H.; Zhao, H. One-step synthesis of cobalt-doped MoS2 nanosheets as bifunctional electrocatalysts for overall water splitting under both acidic and alkaline conditions. Chem. Commun. 2018, 54, 3859–3862. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Liu, C.; Dai, C. Ni3S2/carbon nanotube nanocomposite as electrode material for hydrogen evolution reaction in alkaline electrolyte and enzyme-free glucose detection. Appl. Catal. B 2014, 154, 213–220. [Google Scholar] [CrossRef]
- Shi, H.; Liang, H.; Ming, F.; Wang, Z. Efficient overall water-splitting electrocatalysis using lepidocrocite VOOH hollow nanospheres. Angew. Chem. Int. Ed. Engl. 2017, 56, 573–577. [Google Scholar] [CrossRef]
- Sun, H.; Lian, Y.; Yang, C.; Xiong, L.; Qi, P.; Mu, Q.; Zhao, X.; Guo, J.; Deng, Z.; Peng, Y. A hierarchical nickel–carbon structure templated by metal–organic frameworks for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 2363–2371. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.; Perry, E.; Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef]
- Wang, P.; Pu, Z.; Li, W.; Zhu, J.; Zhang, C.; Zhao, Y.; Mu, S. Coupling NiSe2-Ni2P heterostructure nanowrinkles for highly efficient overall water splitting. J. Catal. 2019, 377, 600–608. [Google Scholar] [CrossRef]
- Du, Y.; Cheng, G.; Luo, W. NiSe2/FeSe2 nanodendrites: A highly efficient electrocatalyst for oxygen evolution reaction. Catal. Sci. Technol. 2017, 7, 4604–4608. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Xu, K.; Wei, Z.; Li, H.; Zhang, T.; Li, X.; Cai, W.; Ma, J.; Fan, H.; Li, Y. Strong electronic interaction in dual-cation-incorporated NiSe2 nanosheets with lattice distortion for highly efficient overall water splitting. Adv. Mater. 2018, 30, 1802121. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Feng, X. Interface engineering of MoS2/Ni3S2 heterostructures for highly enhanced electrochemical overall-water-splitting activity. Angew. Chem. Int. Ed. Engl. 2016, 55, 6702–6707. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Zhou, L.; Gu, S.; Gao, H.; Ren, X.; Li, S.; Wang, R.; Guo, H. A subtle functional design of hollow CoP@MoS2 hetero-nanoframes with excellent hydrogen evolution performance. Mater. Des. 2021, 211, 110165. [Google Scholar] [CrossRef]
- Wang, K.; Paulus, B. Cluster formation effect of water on pristine and defective MoS2 monolayers. Nanomaterials 2023, 13, 229. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Zhang, H.; He, Y.; Liu, W.; Wang, R. Interface structure and strain controlled Pt nanocrystals grown at side facet of MoS2 with critical size. Nano Res. 2022, 15, 8493–8501. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.; Bonde, J.; Jørgensen, K.; Nielsen, J.; Horch, S.; Chorkendorff, I.; Nørskov, J. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Xiao, Y.; Wu, W.; Zhang, X.; Fu, Q.; Zhao, Y.; Qu, L. All-pH-tolerant in-plane heterostructures for efficient hydrogen evolution reaction. ACS Nano 2021, 15, 11417–11427. [Google Scholar] [CrossRef] [PubMed]
- Irzhak, A.; Irzhak, D.; Kononenko, O.; Pundikov, K.; Roshchupkin, D. Changes in the Raman spectrum of monolayer graphene under compression/stretching strain in graphene/piezoelectric crystal structures. Nanomaterials 2023, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, H.; Li, J. Graphene and graphene-like layered transition metal dichalcogenides in energy conversion and storage. Small 2014, 10, 2165–2181. [Google Scholar] [CrossRef]
- Meng, X.; Yu, L.; Ma, C.; Nan, B.; Si, R.; Tu, Y.; Deng, J.; Deng, D.; Bao, X. Three-dimensionally hierarchical MoS2/graphene architecture for high-performance hydrogen evolution reaction. Nano Energy 2019, 61, 611–616. [Google Scholar] [CrossRef]
- Shan, A.; Teng, X.; Zhang, Y.; Zhang, P.; Xu, Y.; Liu, C.; Li, H.; Ye, H.; Wang, R. Interfacial electronic structure modulation of Pt-MoS2 heterostructure for enhancing electrocatalytic hydrogen evolution reaction. Nano Energy 2022, 94, 106913. [Google Scholar] [CrossRef]
- Jeghan, S.; Kim, D.; Lee, Y.; Kim, M.; Lee, G. Designing a smart heterojunction coupling of cobalt-iron layered double hydroxide on nickel selenide nanosheets for highly efficient overall water splitting kinetics. Appl. Catal. B 2022, 308, 121221. [Google Scholar] [CrossRef]
- Oyetade, O.; Kriek, R. NiSe-Ni3Se2/multiwalled carbon nanotube composites as efficient electrocatalysts for the oxygen evolution reaction in alkaline media. Electrocatalysis 2019, 11, 35–45. [Google Scholar] [CrossRef]
- Kwak, I.; Im, H.; Jang, D.; Kim, Y.; Park, K.; Lim, Y.; Cha, E.; Park, J. CoSe2 and NiSe2 nanocrystals as superior bifunctional catalysts for electrochemical and photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2016, 8, 5327–5334. [Google Scholar] [CrossRef]
- Zhou, J.; Yuan, L.; Wang, J.; Song, L.; You, Y.; Zhou, R.; Zhang, J.; Xu, J. Combinational modulations of NiSe2 nanodendrites by phase engineering and iron-doping towards an efficient oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 8113–8120. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Li, J.; Lin, X.; Li, X.; Fang, Y.; Huang, J.; Li, W.; Tian, M.; Jin, J.; et al. Synthesis of Cu–MoS2/rGO hybrid as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Power Sources 2015, 292, 15–22. [Google Scholar] [CrossRef]
- Li, Z.; Ma, X.; Wu, L.; Ye, H.; Li, L.; Lin, S.; Zhang, X.; Shao, Z.; Yang, Y.; Gao, H. Synergistic effect of cocatalytic NiSe2 on stable 1T-MoS2 for hydrogen evolution. RSC Adv. 2021, 11, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Ge, C.; Xing, Z.; Asiri, A.; Sun, X. NixSy-MoS2 hybrid microspheres: One-pot hydrothermal synthesis and their application as a novel hydrogen evolution reaction electrocatalyst with enhanced activity. Electrochim. Acta 2014, 137, 504–510. [Google Scholar] [CrossRef]
- Nai, J.; Xu, X.; Xie, Q.; Lu, G.; Wang, Y.; Luan, D.; Tao, X.; Lou, X. Construction of Ni(CN)2/NiSe2 heterostructures by stepwise topochemical pathways for efficient electrocatalytic oxygen evolution. Adv. Mater. 2022, 34, 2104405. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Li, Z.; Xue, K.; Ruan, Y.; Ao, X.; Wan, H.; Miao, X.; Zhang, B.; Jiang, J.; Wang, C.; et al. Tailoring the electrocatalytic activity of bimetallic nickel-iron diselenide hollow nanochains for water oxidation. Nano Energy 2018, 47, 275–284. [Google Scholar] [CrossRef]
- Ding, S.; He, P.; Feng, W.; Li, L.; Zhang, G.; Chen, J.; Dong, F.; He, H. Novel molybdenum disulfide nanosheets–decorated polyaniline: Preparation, characterization and enhanced electrocatalytic activity for hydrogen evolution reaction. J. Phys. Chem. Solids 2016, 91, 41–47. [Google Scholar] [CrossRef]
- Sandoval, S.; Yang, D.; Frindt, R.; Irwin, J. Raman study and lattice dynamics of single molecular layers of MoS2. Phys. Rev. B Condens. Matter Mater. Phys. 1991, 44, 3955–3962. [Google Scholar] [CrossRef]
- Gao, X.; Qi, J.; Wan, S.; Zhang, W.; Wang, Q.; Cao, R. Conductive molybdenum sulfide for efficient electrocatalytic hydrogen evolution. Small 2018, 14, 1803361. [Google Scholar] [CrossRef]
- Zhai, L.; Lo, T.; Xu, Z.; Potter, J.; Mo, J.; Guo, X.; Tang, C.; Tsang, S.; Lau, S. In situ phase transformation on nickel-based selenides for enhanced hydrogen evolution reaction in alkaline medium. ACS Energy Lett. 2020, 5, 2483–2491. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Xu, C.; Chen, N.; Li, Y.; Liu, H.; Zhen, L.; Dravid, V.; Wu, J. NiSe2 pyramids deposited on N-doped graphene encapsulated Ni foam for high-performance water oxidation. J. Mater. Chem. A 2017, 5, 3981–3986. [Google Scholar] [CrossRef]
- Liang, M.; Xia, T.; Gao, H.; Zhao, K.; Cao, T.; Deng, M.; Ren, X.; Li, S.; Guo, H.; Wang, R. Modulating reaction pathways of formic acid oxidation for optimized electrocatalytic performance of PtAu/CoNC. Nano Res. 2021, 15, 1221–1229. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Qian, H.; Wang, Z.; Yamauchi, Y. One-pot synthesis of zeolitic imidazolate framework 67-derived hollow Co3S4@MoS2 heterostructures as efficient bifunctional catalysts. Chem. Mater. 2017, 29, 5566–5573. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Gao, T.; Yao, T.; Zhang, X.; Han, J.; Wang, X.; Zhang, Z.; Xu, P.; Zhang, P.; et al. Synergistic phase and disorder engineering in 1T-MoSe2 nanosheets for enhanced hydrogen-evolution reaction. Adv. Mater. 2017, 29, 1700311. [Google Scholar] [CrossRef]
- Liu, H.; Xie, R.; Luo, Y.; Cui, Z.; Yu, Q.; Gao, Z.; Zhang, Z.; Yang, F.; Kang, X.; Ge, S.; et al. Dual interfacial engineering of a chevrel phase electrode material for stable hydrogen evolution at 2500 mA cm−2. Nat. Commun. 2022, 13, 6382. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Guan, Y.; Zhou, E.; Zhang, X.; Wang, Y. Nanoscale double-heterojunctional electrocatalyst for hydrogen evolution. Adv. Sci. 2022, 9, 2201339. [Google Scholar] [CrossRef]
- Sun, Q.; Tong, Y.; Chen, P.; Zhou, B.; Dong, X. Universal strategy of bimetal heterostructures as superior bifunctional catalysts for electrochemical water splitting. ACS Sustain. Chem. Eng. 2021, 9, 4206–4212. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.; Wang, S.; Ding, D.; Chen, M.; Liu, C.; Tian, Z.; Novoselov, K.; Ma, C.; Deng, D.; et al. Multiscale structural and electronic control of molybdenum disulfide foam for highly efficient hydrogen production. Nat. Commun. 2017, 8, 14430. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Sun, K.; Wang, X.; Liu, Y.; Pan, Y.; Liu, Z.; Cao, D.; Song, Y.; Liu, S.; Liu, C. Three-dimensional-networked Ni2P/Ni3S2 heteronanoflake arrays for highly enhanced electrochemical overall-water-splitting activity. Nano Energy 2018, 51, 26–36. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X. Construction of hierarchical Ni–Co–P hollow nanobricks with oriented nanosheets for efficient overall water splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhang, C.; Gao, H.; Lv, L.; Han, L.; Zhang, Z. Self-supported porous NiSe2 nanowrinkles as efficient bifunctional electrocatalysts for overall water splitting. ACS Sustain. Chem. Eng. 2017, 6, 2231–2239. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Pang, Q.; Zhang, J. Core/shell cable-like Ni3S2 nanowires/N-doped graphene-like carbon layers as composite electrocatalyst for overall electrocatalytic water splitting. Chem. Eng. J. 2020, 401, 126045. [Google Scholar] [CrossRef]
- An, L.; Feng, J.; Zhang, Y.; Wang, R.; Liu, H.; Wang, G.; Cheng, F.; Xi, P. Epitaxial heterogeneous interfaces on N-NiMoO4/NiS2 nanowires/nanosheets to boost hydrogen and oxygen production for overall water splitting. Adv. Funct. Mater. 2019, 29, 1805298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xia, X.; Cao, X.; Zhang, B.; Tiep, N.; He, H.; Chen, S.; Huang, Y.; Fan, H. Ultrafine metal nanoparticles/N-doped porous carbon hybrids coated on carbon fibers as flexible and binder-free water splitting catalysts. Adv. Energy Mater. 2017, 7, 1700220. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Hansen, L.; Nørskov, J. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Nørskov, J.; Bligaard, T.; Logadottir, A.; Kitchin, J.; Chen, J.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. Phys. Inorg. Chem. 2005, 152, J23–J26. [Google Scholar] [CrossRef] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Cao, T.; Xia, T.; Wu, C.; Feng, M.; Li, X.; Mei, Z.; Gao, H.; Huo, D.; Ren, X.; et al. MoS2/NiSe2/rGO Multiple-Interfaced Sandwich-like Nanostructures as Efficient Electrocatalysts for Overall Water Splitting. Nanomaterials 2023, 13, 752. https://doi.org/10.3390/nano13040752
Bai X, Cao T, Xia T, Wu C, Feng M, Li X, Mei Z, Gao H, Huo D, Ren X, et al. MoS2/NiSe2/rGO Multiple-Interfaced Sandwich-like Nanostructures as Efficient Electrocatalysts for Overall Water Splitting. Nanomaterials. 2023; 13(4):752. https://doi.org/10.3390/nano13040752
Chicago/Turabian StyleBai, Xiaoyan, Tianqi Cao, Tianyu Xia, Chenxiao Wu, Menglin Feng, Xinru Li, Ziqing Mei, Han Gao, Dongyu Huo, Xiaoyan Ren, and et al. 2023. "MoS2/NiSe2/rGO Multiple-Interfaced Sandwich-like Nanostructures as Efficient Electrocatalysts for Overall Water Splitting" Nanomaterials 13, no. 4: 752. https://doi.org/10.3390/nano13040752