A Comparison between Porous to Fully Dense Electrodeposited CuNi Films: Insights on Electrochemical Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CuNi Films
2.2. Characterization
2.3. Electrocatalytic Activity towards HER
3. Results
3.1. Morphology and Structure of CuNi Films
3.2. Surface Roughness
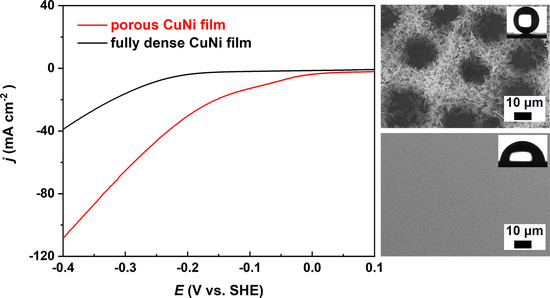
3.3. Electrocatalytic Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Egorov, V.; O’Dwyer, C. Architected porous metals in electrochemical energy storage. Curr. Opin. Electrochem. 2020, 21, 201–208. [Google Scholar] [CrossRef]
- Lu, B.; Wang, Y.; Li, W.; Song, S.; Tian, P.; Li, R.; Tian, X.; Liu, X.; Zang, J. Ni-P alloy@carbon nanotubes immobilized on the framework of Ni foam as a 3D hierarchical porous self-supporting electrode for hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 23245–23253. [Google Scholar] [CrossRef]
- Park, Y.S.; Choi, W.S.; Jang, M.J.; Lee, J.H.; Park, S.; Jin, H.; Seo, M.H.; Lee, K.H.; Yin, Y.; Kim, Y.; et al. Three-dimensional dendritic Cu-Co-P electrode by one-step electrodeposition on a hydrogen bubble template for hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2019, 7, 10734–10741. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Guo, Y.; Hu, A.; Li, M.; Hang, T.; Ling, H. 3D hierarchical nanostructured Ni-Co alloy electrodes on porous nickel for hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 29946–29955. [Google Scholar] [CrossRef]
- Sun, H.; Tian, C.; Li, Y.; Wu, J.; Wang, Q.; Yan, Z.; Li, C.P.; Cheng, F.; Du, M. Coupling NiCo alloy and CeO2 to enhance electrocatalytic hydrogen evolution in alkaline solution. Adv. Sustain. Syst. 2020, 4, 2000122. [Google Scholar] [CrossRef]
- Wang, J.; Chang, K.; Sun, Z.; Lee, J.H.; Tackett, B.M.; Zhang, C.; Chen, J.G.; Liu, C.J. A combined experimental and theoretical study of the accelerated hydrogen evolution kinetics over wide pH range on porous transition metal doped tungsten phosphide electrocatalysts. Appl. Catal. B-Environ. 2019, 251, 162–167. [Google Scholar] [CrossRef]
- Hao, S.; Liu, J.; Cao, Q.; Zhao, Y.; Zhao, X.; Pei, K.; Zhang, J.; Chen, G.; Che, R. In-situ electrochemical pretreatment of hierarchical Ni3S2-based electrocatalyst towards promoted hydrogen evolution reaction with low overpotential. J. Colloid Interface Sci. 2020, 559, 282–290. [Google Scholar] [CrossRef]
- Li, C.; Iqbal, M.; Jiang, B.; Wang, Z.; Kim, J.; Nanjundan, A.K.; Whitten, A.E.; Wood, K.; Yamauchi, Y. Pore-tuning to boost the electrocatalytic activity of polymeric micelle-templated mesoporous Pd nanoparticles. Chem. Sci. 2019, 10, 4054–4061. [Google Scholar] [CrossRef] [Green Version]
- Malgras, V.; Ataee-esfahani, H.; Wang, H.; Jiang, B.; Li, C.; Wu, K.C.-W.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for mesoporous metals. Adv. Mater. 2016, 28, 993–1010. [Google Scholar] [CrossRef]
- Chen, A.Y.; Wang, J.W.; Jia, Y.Q.; Jia, Y.Q.; Gu, J.F.; Xie, X.F.; Pan, D. Effects of pore size and residual Ag on electrocatalytic properties of nanoporous gold films prepared by pulse electrochemical dealloying. Electrochim. Acta 2015, 153, 552–558. [Google Scholar] [CrossRef]
- Li, C.; Jiang, B.; Chen, H.; Imura, M.; Sang, L.; Malgras, V.; Bando, Y.; Ahamad, T.; Alshehri, S.M.; Tominaka, S.; et al. Superior electrocatalytic activity of mesoporous Au film templated from diblock copolymer micelles. Nano Res. 2016, 9, 1752–1762. [Google Scholar] [CrossRef]
- Wei, L.; Goh, K.; Birer, O.; Karahan, H.E.; Chang, J.; Zhai, S.; Chen, X.; Chen, Y. A hierarchically porous nickel-copper phosphide nano-foam for efficient electrochemical splitting of water. Nanoscale 2017, 9, 4401–4408. [Google Scholar] [CrossRef]
- Ji, S.M.; Muthurasu, A.; Chhetri, K.; Kim, H.Y. Metal-organic framework assisted vanadium oxide nanorods as efficient electrode materials for water oxidation. J. Colloid Interface Sci. 2022, 618, 475–482. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Pan, Z.; Liu, L.; Xi, J.; Luo, X.; Shen, Y. Exceptional performance of hierarchical Ni-Fe (hydr)oxide@NiCu electrocatalysts for water splitting. Adv. Mater. 2019, 31, 1806769. [Google Scholar] [CrossRef]
- Santos, H.L.S.; Corradini, P.G.; Medina, M.; Dias, J.A.; Mascaro, L.H. NiMo-NiCu inexpensive composite with high activity for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2020, 12, 17492–17501. [Google Scholar] [CrossRef]
- Guo, X.; Liang, T.; Zhang, D.; Zhang, M.; Lin, Y.; Lai, C. Facile fabrication of 3D porous nickel networks for electro-oxidation of methanol and ethanol in alkaline medium. Mater. Chem. Phys. 2019, 221, 390–396. [Google Scholar] [CrossRef]
- Li, D.; Podlaha, E.J. Template-assisted electrodeposition of porous Fe-Ni-Co nanowires with vigorous hydrogen evolution. Nano Lett. 2019, 19, 3569–3674. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Wang, Z.; Li, Y.; Xu, Y.; Li, X.; Xue, H.; Wang, L. Electrochemical fabrication of porous Au film on Ni foam for nitrogen reduction to ammonia. Small 2019, 15, 1804769. [Google Scholar] [CrossRef]
- Cherevko, S.; Xing, X.; Chung, C. Hydrogen template assisted electrodeposition of sub-micrometer wires composing honeycomb-like porous Pb films. Appl. Surf. Sci. 2011, 257, 8054–8061. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, C.; Wang, A.; Zeng, Y. 3-D network pore structures in copper foams by electrodeposition and hydrogen bubble templating mechanism. J. Electrochem. Soc. 2015, 162, D365–D370. [Google Scholar] [CrossRef]
- Zhang, J.; Baro, M.D.; Pellicer, E.; Sort, J. Electrodeposition of magnetic, superhydrophobic, non-stick, two-phase Cu-Ni foam films and their enhanced performance for hydrogen evolution reaction in alkaline water media. Nanoscale 2014, 6, 12490–12499. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, J.; Kim, Y.; Kim, G.P.; Baeck, S.H. Electrosynthesis of mesoporous Pt-Au alloy electrode for direct methanol fuel cell. J. Phys. Chem. Solids 2008, 69, 1284–1287. [Google Scholar] [CrossRef]
- Ojani, R.; Hasheminejad, E.; Raoof, J. Hydrogen evolution assisted electrodeposition of bimetallic 3D nano/micro-porous PtPd films and their electrocatalytic performance. Int. J. Hydrogen Energy 2014, 39, 8194–8203. [Google Scholar] [CrossRef]
- Lange, G.A.; Eugenio, S.; Duarte, R.G.; Silva, T.M.; Carmezim, M.J. Characterisation and electrochemical behaviour of electrodeposited Cu-Fe foams applied as pseudocapacitor electrodes. J. Electroanal. Chem. 2015, 737, 85–92. [Google Scholar] [CrossRef]
- Kang, K.; Kim, S.; Yoon, J.; Kim, J.; Cahoon, C.; Jang, J. Bi-functional 3D-NiCu-double hydroxide@partially etched 3D-NiCu catalysts for non-enzymatic glucose detection and the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2022, 14, 33013–33023. [Google Scholar] [CrossRef]
- Asnavandi, M.; Suryanto, B.H.R.; Yang, W.; Bo, X.; Zhao, C. Dynamic hydrogen bubble templated NiCu phosphide electrodes for pH-insensitive hydrogen evolution reactions. ACS Sustain. Chem. Eng. 2018, 6, 2866–2871. [Google Scholar] [CrossRef]
- Solmz, R.; Doner, A.; Kardas, G. The stability of hydrogen evolution activity and corrosion behavior of NiCu coatings with long-term electrolysis in alkaline solution. Int. J. Hydrogen Energy 2009, 34, 2089–2094. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Nag, N.; Crozier, P.A. In situ Preparation of Ni–Cu/TiO2 Bimetallic Catalysts. J. Catal. 2009, 262, 73–82. [Google Scholar] [CrossRef]
- Best, R.J.; Russel, W.W. Nickel, Copper and Some of their Alloys as Catalysts for Ethylene Hydrogenation. J. Am. Chem. Soc. 1959, 81, 4132–4137. [Google Scholar]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Gerbasi, R.; Guerriero, P.; Musiani, M.; Vazquez-Gomez, L.; Verlato, E. Electrodeposition of Cu-Ni alloy electrodes with bimodal porosity and their use for nitrate reduction. ECS Electrochem. Lett. 2013, 2, D58–D60. [Google Scholar] [CrossRef]
- Solmz, R.; Done, A.; Kardas, G. Electrochemical deposition and characterization of NiCu coatings as cathode materials for hydrogen evolution reaction. Electrochem. Commun. 2008, 10, 1909–1911. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, J.; Eiler, K.; Yang, Z.; Fan, L.; Yang, D.; Zhang, M.; Hou, Y.; Guan, R.; Sort, J.; et al. Electrochemically fabricated surface-mesostructured CuNi bimetallic catalysts for hydrogen production in alkaline media. Nanomaterials 2022, 12, 118. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Butyrina, T.E.; Makhota, D.O.; Korniy, S.A.; Danilov, F.I. Anodic treatment of Ni-Cu alloy in a deep eutectic solvent to improve electrocatalytic activity in the hydrogen evolution reaction. Port. Electrochimica Acta 2023, 41, 29–45. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Butyrina, T.E.; Makhota, D.O.; Korniy, S.A.; Danilov, F.I. Electrochemical treatment of nickel-copper alloy in ionic liquid based on eutectic mixture of choline chloride and ethylene glycol as a way to increase electrocatalytic activity towards hydrogen evolution reaction. Funct. Mater. 2022, 29, 93–99. [Google Scholar]
- Pellicer, E.; Varea, A.; Pane, S.; Nelson, B.J.; Menendez, E.; Estrader, M.; Surinach, S.; Baro, M.D.; Nogues, J.; Sort, J. Nanocrystalline electroplated Cu-Ni: Metallic thin films with enhanced mechanical properties and tunable magnetic behavior. Adv. Funct. Mater. 2010, 20, 983–991. [Google Scholar] [CrossRef]
- Pellicer, E.; Varea, A.; Pane, S.; Sivaraman, K.M.; Nelson, B.J.; Surinach, S.; Baro, M.D.; Sort, J. A comparison between fine-grained and nanocrystalline electrodeposited Cu-Ni films. Insights on mechanical and corrosion performance. Sur. Coat. Tech. 2011, 205, 5285–5293. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Chen, W. Saccharin effects on direct-current electroplating nanocrystalline Ni-Cu alloys. J. Electrochem. Soc. 2008, 155, K133–K139. [Google Scholar] [CrossRef]
- Eugénio, S.; Silva, T.M.; Carmezim, M.J.; Guarte, R.G.; Montemor, M.F. Electrodeposition and characterization of nickel–copper metallic foams for application as electrodes for supercapacitors. J. Appl. Electrochem. 2014, 44, 455–465. [Google Scholar] [CrossRef]
- Hiraishi, N.; Gondo, T.; Shimada, Y.; Hill, R.; Hayashi, F. Crystallographic and physicochemical analysis of bovine and human teeth using X-ray diffraction and solid-state nuclear magnetic resonance. J. Funct. Biomater. 2022, 13, 254. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, L.; Chien, C.; Searson, P.C. Formation of a core/shell microstructure in Cu-Ni thin films. J. Electrochem. Soc. 2008, 155, D569–D574. [Google Scholar] [CrossRef]
- Choi, W.; Jung, H.; Kwon, S.; Lee, J.W.; Liu, M.; Shin, H.C. Nanostructured metallic foam electrodeposits on a nonconductive substrate. J. Mater. Chem. 2012, 22, 1028–1032. [Google Scholar] [CrossRef]
- Song, X.; Tang, H.; Zhang, Y.; Zheng, S.X. Sampling optimization for 3D surface measurement. Part I: Sampling area optimization based on areal texture parameter analysis. Measurement 2022, 203, 111972. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, X.; Eikerling, M. The rate-determining term of electrocatalytic reactions with first-order kinetics. Electrochim. Acta 2021, 393, 139019. [Google Scholar] [CrossRef]
- Chua, C.; Ansovini, D.; Lee, C.; Teng, Y.T.; Ong, L.T.; Chim, D.; Andy, T.S.; Raja, R.; Lim, Y.F. The effect of crystallinity on photocatalytic performance Co3O4 water splitting cocatalysts. Phys. Chem. Chem. Phys. 2016, 18, 5172–5178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellenberger, A.; Vaszilcsin, N.; Brandl, W.; Duteanu, N. Kinetics of hydrogen evolution reaction on skeleton nickel and nickel-titanium electrodes obtained by thermal arc spraying technique. Int. J Hydrogen energy 2007, 32, 3258–3265. [Google Scholar] [CrossRef]
- Liu, X.M.; Juan, L.; Zhan, L.; Tang, L.; Wang, Y.L.; Qiao, W.M.; Liang, X.Y.; Ling, L.C. Effect of conductive filler on the impedance behaviors of activated carbon based electric double layer capacitors. J. Electroanal. Chem. 2010, 642, 75–81. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Bo, X.; Wang, F. Highly active porous nickel-film electrode via polystyrene microsphere template-assisted composite electrodeposition for hydrogen-evolution reaction in alkaline medium. Sci. China Chem. 2015, 58, 501–507. [Google Scholar] [CrossRef]
- Jin, J.; Ge, J.; Zhao, X.; Wang, Y.; Zhang, F.; Lei, X. An amorphous NiCuFeP@Cu3P nanoarray for an efficient hydrogen evolution reaction. Inorg. Chem. Front. 2022, 9, 1446–1455. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Solmaz, R.; Yuksel, H. Fabrication, characterization and application of three-dimensional copper nanodomes as efficient cathodes for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 14108–14116. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, J.; Jo, S.; Kim, S.; Park, K.; Kim, S.; Han, H.; Paik, U.; Song, T. Highly efficient and stable bifunctional electrocatalysts with decoupled active sites for hydrogen evolution and oxygen reduction reactions. Appl. Catal. B-Environ. 2021, 298, 120530. [Google Scholar] [CrossRef]











| PorousCuNi | Ni phase d = 2.015 a = b = c = 3.53181 at% Ni = 27% average crystallite size 60 nm | Cu phase d = 2.064 a = b = c = 3.61597 at% Cu = 73% average crystallite size 55 nm | Rp (%) = 4.03 Rwp (%) = 5.71 Re (%) = 5.35 GOF = 1.07 |
| Fully dense CuNi | d = 2.044 a = b = c = 3.55516 at% Ni = 46% at% Cu = 54% average crystallite size 32 nm | Rp (%) = 2.10 Rwp (%) = 2.78 Re (%) = 1.70 GOF = 1.63 |
| Sample | Porous CuNi Film | Fully Dense CuNi Film |
|---|---|---|
| Rs (Ω cm2) | 1.50 | 2.06 |
| Q1 (Sn Ω−1 cm−2) | 1.58 × 10−3 | 5.04 × 10−5 |
| n1 | 0.90 | 0.87 |
| R1 (Ω cm2) | 39.75 | 121.50 |
| Q2 (Sn Ω−1 cm−2) | 1.03 × 10−2 | 1.20 × 10−3 |
| n2 | 0.82 | 0.71 |
| R2 (Ω cm2) | 104.90 | 237.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Bai, J.; Zhang, M.; Chen, Y.; Fan, L.; Yang, Z.; Zhang, J.; Guan, R. A Comparison between Porous to Fully Dense Electrodeposited CuNi Films: Insights on Electrochemical Performance. Nanomaterials 2023, 13, 491. https://doi.org/10.3390/nano13030491
Wang X, Bai J, Zhang M, Chen Y, Fan L, Yang Z, Zhang J, Guan R. A Comparison between Porous to Fully Dense Electrodeposited CuNi Films: Insights on Electrochemical Performance. Nanomaterials. 2023; 13(3):491. https://doi.org/10.3390/nano13030491
Chicago/Turabian StyleWang, Xuejiao, Jingyuan Bai, Meilin Zhang, Yuxi Chen, Longyi Fan, Zhou Yang, Jin Zhang, and Renguo Guan. 2023. "A Comparison between Porous to Fully Dense Electrodeposited CuNi Films: Insights on Electrochemical Performance" Nanomaterials 13, no. 3: 491. https://doi.org/10.3390/nano13030491