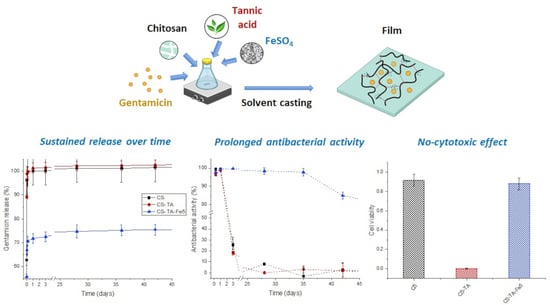
Prolonged Antibacterial Activity in Tannic Acid–Iron Complexed Chitosan Films for Medical Device Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation Procedure
2.3. Films Characterization
2.3.1. Thickness
2.3.2. Swelling in PBS
2.3.3. Mass loss in PBS
2.3.4. Contact Angle
2.3.5. X-Ray Photoelectron Spectroscopy
2.3.6. Fourier-Transform Infrared Spectroscopy
2.4. Antibiotic Release
2.5. Antibacterial Assays
2.5.1. Bacteria Stock Preparation
2.5.2. Disk Diffusion Test
2.5.3. Indirect Antibacterial Activity over Time
2.6. Bicompatibility
2.6.1. Cell Culture
2.6.2. Indirect Cytotoxicity Assay
2.6.3. Hemolysis Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Film Characterization
3.2. Antibiotic Release
3.3. Antibacterial Activity
3.4. Biocompatibility
3.4.1. Indirect Toxicity Assay
3.4.2. Hemocompatibility
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calligaro, K.D. Infections Associated with Indwelling Medical Devices. J. Vasc. Surg. 1995, 21, 1002. [Google Scholar] [CrossRef] [Green Version]
- Health Canada. Evaluation of Healthcare Associated Infection Activities at the Public Health Agency of Canada 2012–13 to 2016–17; Office of Audit and Evaluation, Health Canada and the Public Health Agency of Canada: Ottawa, ON, Canada, 2018. [Google Scholar]
- ODPHP Healthy People 2030. Available online: https://www.healthypeople.gov/2020/topics-objectives/topic/healthcare-associated-infections#4 (accessed on 11 November 2022).
- Van Epps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597. [Google Scholar] [CrossRef] [Green Version]
- Stærk, K.; Grønnemose, R.B.; Palarasah, Y.; Kolmos, H.J.; Lund, L.; Alm, M.; Thomsen, P.; Andersen, T.E. A Novel Device-Integrated Drug Delivery System for Local Inhibition of Urinary Tract Infection. Front. Microbiol. 2021, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Sharma, P.; Vishwamitra, B.; Singh, G. Review on Surface Treatment for Implant Infection via Gentamicin and Antibiotic Releasing Coatings. Coatings 2021, 11, 1006. [Google Scholar] [CrossRef]
- Brianezi, S.F.S.; Castro, K.C.; Piazza, R.D.; do Socorro Fernandes Melo, M.; Pereira, R.M.; Marques, R.F.C.; Campos, M.G.N. Preparation and Characterization of Chitosan/MPEG-PCL Blended Membranes for Wound Dressing and Controlled Gentamicin Release. Mater. Res. 2018, 21, e20170951. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Ruini, F.; Tonda-Turo, C.; Chiono, V.; Ciardelli, G. Chitosan Membranes for Tissue Engineering: Comparison of Different Crosslinkers. Biomed. Mater. 2015, 10, 065002. [Google Scholar] [CrossRef]
- Sabaghi, M.; Tavasoli, S.; Hoseyni, S.Z.; Mozafari, M.R.; Degraeve, P.; Katouzian, I. A Critical Review on Approaches to Regulate the Release Rate of Bioactive Compounds from Biopolymeric Matrices. Food Chem. 2022, 382, 132411. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Crosslinked Chitosan—Preparation and Characterization. Carbohydr. Res. 2006, 341, 169–173. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Nair, P. Physical and Chemical Reinforcement of Chitosan Film Using Nanocrystalline Cellulose and Tannic Acid. Cellulose 2015, 22, 2529–2541. [Google Scholar] [CrossRef]
- Wiggers, H.J.; Chevallier, P.; Copes, F.; Simch, F.H.; da Silva Veloso, F.; Genevro, G.M.; Mantovani, D. Quercetin-Crosslinked Chitosan Films for Controlled Release of Antimicrobial Drugs. Front. Bioeng. Biotechnol. 2022, 10, 814162. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Zhang, Q.; Jin, Z. Tannic Acid-Based Multifunctional Hydrogels with Facile Adjustable Adhesion and Cohesion Contributed by Polyphenol Supramolecular Chemistry. ACS Omega 2017, 2, 6668–6676. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B.; Mazur, O.; Miłek, O.; Michalska-Sionkowska, M.; Osyczka, A.M.; Kleszczyński, K. Development of Tannic Acid-Enriched Materials Modified by Poly(ethylene glycol) for Potential Applications as Wound Dressing. Prog. Biomater. 2020, 9, 115–123. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Wang, W.; Gai, C.; Zhang, W.; Li, W.; Ding, D. Fe(II) and Tannic Acid-Cloaked MOF as Carrier of Artemisinin for Supply of Ferrous Ions to Enhance Treatment of Triple-Negative Breast Cancer. Nanoscale Res. Lett. 2021, 16, 37. [Google Scholar] [CrossRef]
- Vučićevic-Prcetic, K.; Cservenák, R.; Radulović, N. Development and Validation of Liquid Chromatography Tandem Mass Spectrometry Methods for the Determination of Gentamicin, Lincomycin, and Spectinomycin in the Presence of Their Impurities in Pharmaceutical Formulations. J. Pharm. Biomed. Anal. 2011, 56, 736–742. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, R. Study of Complexes of Tannic Acid with Fe(III) and Fe(II). J. Anal. Methods Chem. 2019, 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Kaczmarek, B.; Owczarek, A.; Nadolna, K.; Sionkowska, A. The Film-Forming Properties of Chitosan with Tannic Acid Addition. Mater. Lett. 2019, 245, 22–24. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A.; Michalska-Sionkowska, M.; Sionkowska, A. The Characterization of Thin Films Based on Chitosan and Tannic Acid Mixture for Potential Applications as Wound Dressings. Polym. Test. 2019, 78, 106007. [Google Scholar] [CrossRef]
- Acharya, V.; Ghosh, A.; Chowdhury, A.R.; Datta, P. Tannic Acid-Crosslinked Chitosan Matrices Enhance Osteogenic Differentiation and Modulate Epigenetic Status of Cultured Cells over Glutaraldehyde Crosslinking. Soft Mater. 2022, 20, 149–160. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Li, Q.; Liu, X.; Guo, Z. Preparation of Cross-Linked Chitosan Quaternary Ammonium Salt Hydrogel Films Loading Drug of Gentamicin Sulfate for Antibacterial Wound Dressing. Mar. Drugs 2021, 19, 479. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Harte, B.R. Physical Properties and Antioxidant Activity of an Active Film from Chitosan Incorporated with Green Tea Extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Rosenkrantz, B.E.; Greco, J.R.; Hoogerheide, J.G.; Oden, E.M. Gentamicin Sulfate. Anal. Profiles Drug Subst. 1981, 9, 295–340. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH Vibrational Groups in Polysaccharide-Nanocomposite Interactions: A FTIR-ATR Study on Chitosan and Chitosan/Clay Films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Gadzala-Kopciuch, R. Gentamicin Release from Chitosan and Collagen Composites. J. Drug Deliv. Sci. Technol. 2016, 35, 353–359. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, N.; Tang, Z.; Yu, Y.; Hu, Q.; Feng, C. A Study of the Mechanism of Fluoride Adsorption from Aqueous Solutions onto Fe-Impregnated Chitosan. Phys. Chem. Chem. Phys. 2015, 17, 12041–12050. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and Its Derivatives for Controlled Drug Release Applications—A Review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Vasile, B.S.; Oprea, O.; Voicu, G.; Ficai, A.; Andronescu, E.; Teodorescu, A.; Holban, A. Synthesis and Characterization of a Novel Controlled Release Zinc Oxide/Gentamicin-Chitosan Composite with Potential Applications in Wounds Care. Int. J. Pharm. 2014, 463, 161–169. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated Infections, Medical Devices and Biofilms: Risk, Tolerance and Control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST Disk Diffusion Antimicrobial Susceptibility Testing Method and Its Implementation in Routine Microbiology Laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.; Lo, J.; Hsu, E.; Burt, H.M.; Shademani, A.; Lange, D. The Combined Use of Gentamicin and Silver Nitrate in Bone Cement for a Synergistic and Extended Antibiotic Action against Gram-Positive and Gram-Negative Bacteria. Materials 2021, 14, 3413. [Google Scholar] [CrossRef]
- Neut, D.; Kluin, O.S.; Crielaard, B.J.; Van Der Mei, H.C.; Busscher, H.J.; Grijpma, D.W. A Biodegradable Antibiotic Delivery System Based on Poly-(trimethylene carbonate) for the Treatment of Osteomyelitis. Acta Orthop. 2009, 80, 514–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peles, Z.; Binderman, I.; Berdicevsky, I.; Zilberman, M. Soy Protein Films for Wound-Healing Applications: Antibiotic Release, Bacterial Inhibition and Cellular Response. J. Tissue Eng. Regen. Med. 2013, 7, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Zander, Z.K.; Becker, M.L. Antimicrobial and Antifouling Strategies for Polymeric Medical Devices. ACS Macro Lett. 2018, 7, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Pugazhendhi, A.; Vasantharaj, S.; Sathiyavimal, S.; Raja, R.K.; Karuppusamy, I.; Narayanan, M.; Kandasamy, S.; Brindhadevi, K. Organic and Inorganic Nanomaterial Coatings for the Prevention of Microbial Growth and Infections on Biotic and Abiotic Surfaces. Surf. Coat. Technol. 2021, 425, 127739. [Google Scholar] [CrossRef]
- Steinbach, G.; Crisan, C.; Ng, S.L.; Hammer, B.K.; Yunker, P.J. Accumulation of Dead Cells from Contact Killing Facilitates Coexistence in Bacterial Biofilms. J. R. Soc. Interface 2020, 17, 20200486. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The Toxic Effect of Silver Ions and Silver Nanoparticles towards Bacteria and Human Cells Occurs in the Same Concentration Range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, W.; Lu, J.; Guo, X.; Xu, J.; Xu, W.; Chi, Y.; Takuya, N.; Wu, H.; Zhao, L. Tannic Acid-Based Metal Phenolic Networks for Bio-Applications: A Review. J. Mater. Chem. B 2021, 9, 4098–4110. [Google Scholar] [CrossRef]
- Soylu, H.M.; Chevallier, P.; Copes, F.; Ponti, F.; Candiani, G.; Yurt, F.; Mantovani, D. A Novel Strategy to Coat Dopamine-Functionalized Titanium Surfaces With Agarose-Based Hydrogels for the Controlled Release of Gentamicin. Front. Cell. Infect. Microbiol. 2021, 11, 678081. [Google Scholar] [CrossRef] [PubMed]







| Film Composition | CS (mL) | TA (mL) | FeSO4 (mL) | G (mL) | H2O (mL) | |
|---|---|---|---|---|---|---|
| CS | - | 6.67 | 0.0 (0%) | 0.00 (0%) | 1.0 (10%) | 2.33 |
| Fe1 | 6.67 | 0.0 (0%) | 0.33 (1%) | 1.0 (10%) | 2.00 | |
| Fe2 | 6.67 | 0.0 (0%) | 0.67 (2%) | 1.0 (10%) | 1.66 | |
| Fe5 | 6.67 | 0.0 (0%) | 1.67 (5%) | 1.0 (10%) | 0.66 | |
| CS-TA | - | 6.67 | 0.4 (20%) | 0.00 (0%) | 1.0 (10%) | 1.93 |
| Fe1 | 6.67 | 0.4 (20%) | 0.33 (1%) | 1.0 (10%) | 1.60 | |
| Fe2 | 6.67 | 0.4 (20%) | 0.67 (2%) | 1.0 (10%) | 1.26 | |
| Fe5 | 6.67 | 0.4 (20%) | 1.67 (5%) | 1.0 (10%) | 0.26 |
| Film Composition | Thickness (µm) | Swelling (%) | Mass Loss (%) | Contact Angle (°) | |
|---|---|---|---|---|---|
| CS | - | 16.8 ± 2.1 | 410.8 ± 62.4 | 34.9 ± 1.9 | 106 ± 5 |
| Fe1 | 17.4 ± 1.7 | 418.2 ± 86.5 | 31.2 ± 0.4 | 105 ± 5 | |
| Fe2 | 16.4 ± 2.1 | 253.2 ± 14.0 | 38.5 ± 2.9 | 116 ± 4 | |
| Fe5 | 16.0 ± 2.9 | 223.2 ± 50.4 | 34.5 ± 2.8 | 117 ± 6 | |
| CS-TA | - | 18.4 ± 2.2 | 108.7 ± 7.9 | 27.4 ± 1.1 | 96 ± 7 |
| Fe1 | 19.2 ± 2.7 | 169.1 ± 25.7 | 23. 2 ± 1.1 | 107 ± 8 | |
| Fe2 | 18.4 ± 2.9 | 175.5 ± 24.2 | 26.9 ± 1.7 | 102 ± 4 | |
| Fe5 | 18.8 ± 4.9 | 178.0 ± 15.7 | 22.9 ± 0.8 | 102 ± 4 | |
| Film Composition | Atomic Composition * | ||||
|---|---|---|---|---|---|
| %C | %O | %N | %Fe | ||
| CS | - | 68.3 ± 2.0 | 24.5 ± 1.6 | 5.6 ± 0.7 | - |
| Fe1 | 66.4 ± 1.8 | 25.6 ± 1.3 | 7.4 ± 1.4 | 0.4 ± 0.5 | |
| Fe2 | 66.1 ± 0.8 | 25.9 ± 1.0 | 6.7 ± 0.4 | 0.8 ± 0.2 | |
| Fe5 | 62.9 ± 2.0 | 28.6 ± 1.7 | 6.7 ± 0.1 | 1.3 ± 0.2 | |
| CS-TA | - | 64.1 ± 1.2 | 27.8 ± 1.5 | 4.6 ± 0.1 | - |
| Fe1 | 66.3 ± 0.2 | 29.4 ± 0.5 | 4.1 ± 0.7 | - | |
| Fe2 | 64.0 ± 0.8 | 31.0 ± 0.7 | 4.5 ± 0.6 | - | |
| Fe5 | 70.9 ± 0.2 | 24.4 ± 0.1 | 3.0 ± 0.1 | - | |
| Film Formulation | S. aureus (mm) | E. coli (mm) | |
|---|---|---|---|
| CS | - | 25.5 ± 0.7 | 22.0 ± 1.4 |
| Fe1 | 25.0 ± 1.4 | 21.5 ± 0.7 | |
| Fe2 | 23.5 ± 0.7 | 21.5 ± 0.7 | |
| Fe5 | 22.0 ± 1.4 | 20.0 ± 0.0 | |
| CS-TA | - | 24.0 ± 1.4 | 22.0 ± 1.4 |
| Fe1 | 24.5 ± 0.7 | 21.0 ± 0.0 | |
| Fe2 | 22.0 ± 1.4 | 21.5 ± 0.7 | |
| Fe5 | 22.0 ± 1.4 | 20.5 ± 0.7 | |
| Negative control | 0 | 0 | |
| Gentamicin (10 µg) | 21.5 ± 0.7 | 18.5 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevallier, P.; Wiggers, H.J.; Copes, F.; Zorzi Bueno, C.; Mantovani, D. Prolonged Antibacterial Activity in Tannic Acid–Iron Complexed Chitosan Films for Medical Device Applications. Nanomaterials 2023, 13, 484. https://doi.org/10.3390/nano13030484
Chevallier P, Wiggers HJ, Copes F, Zorzi Bueno C, Mantovani D. Prolonged Antibacterial Activity in Tannic Acid–Iron Complexed Chitosan Films for Medical Device Applications. Nanomaterials. 2023; 13(3):484. https://doi.org/10.3390/nano13030484
Chicago/Turabian StyleChevallier, Pascale, Helton José Wiggers, Francesco Copes, Cecilia Zorzi Bueno, and Diego Mantovani. 2023. "Prolonged Antibacterial Activity in Tannic Acid–Iron Complexed Chitosan Films for Medical Device Applications" Nanomaterials 13, no. 3: 484. https://doi.org/10.3390/nano13030484