A Conceptual Approach for the Design of New Catalysts for Ammonia Synthesis: A Metal—Support Interactions Review
Abstract
:1. Introduction
2. Thermocatalytic Ammonia Synthesis
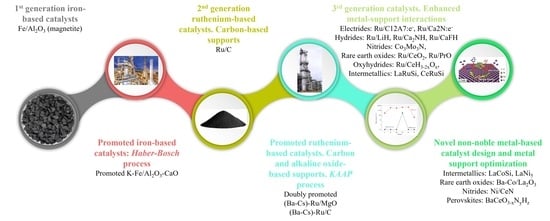
2.1. Historical Evolution of Catalysts for Ammonia Synthesis
2.2. Ammonia Synthesis Reaction Fundamentals
2.3. Ammonia Synthesis Reaction Mechanism
2.4. Metal—Support Interactions: Electron Transfer
| Classification | Catalysts Nature | Catalysts Examples | Industrial Applications 1 | Ref |
|---|---|---|---|---|
| 1st-generation catalysts | Iron-based (magnetite) | Fe/Al2O3 | - | [21] |
| Promoted iron-based | K-Fe/Al2O3-CaO | Haber–Bosch process | [20,21,28,29] | |
| 2nd-generation catalysts | Ruthenium on carbon | Ru/C | - | [22] |
| Doubly promoted ruthenium on non-functional supports | (Ba-Cs)-Ru/MgO (Ba-Cs)-Ru/C | KAAP process | [22] | |
| 3rd-generation catalysts | Electrides | Ru/C12A7:e−, Ru/Ca2N:e− | - | [26,85] |
| Rare earth oxides | Ru/CeO2, Ru/PrO, Ba-Co/La2O3 | [44,74,90] | ||
| Hydrides | Ru/LiH, Ru/Ca2NH, Ru/CaFH | [59,60,87] | ||
| Nitrides | Co3Mo3N, Ni/CeN | [56,91] | ||
| Oxyhydrides (nitrides) | Ru/CeH3-2xOx, BaCeO3-xNyHz | [37,71] | ||
| Intermetallics | LaRuSi, CeRuSi, LaCoSi, LaNi5 | [66,67,73,92] |
| Dissociative Mechanism | Associative Mechanism | |
|---|---|---|
| (1) | N2 (g) + * → N2* | N2 (g) + * → N2* |
| (2) | N2* + * → 2N* | H2 (g) + * → H2* |
| (3) | N* + H* → NH* + * | H2* → 2H* |
| (4) | NH* + H* → NH2* + * | N2* + 2H* → N2H* |
| (5) | NH2* + H* → NH3* + * | N2H* + H* → N2H2* |
| (6) | NH3* → NH3 (g) + * | N2H2* + H* → N2H3* |
| (7) | H2 (g) + * → H2* | N2H3* + H* → N2H4* |
| (8) | H2* → 2H* | N2H4* → 2NH2* |
| (9) | NH2* + H* → NH3* | |
| (10) | NH3* → NH3 (g) + * |
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alfa Laval HHTVSG. Ammonfuel—An Industrial View of Ammonia as a Marine Fuel; Alfa Laval HHTVSG: Nuremberg, Germany, 2020. [Google Scholar]
- Ammonia Global Market Report 2023. Available online: https://www.reportlinker.com/p06323489/Ammonia-Global-Market-Report.html?utm_source=GNW (accessed on 20 June 2023).
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Reese, M.; Marquart, C.; Malmali, M.; Wagner, K.; Buchanan, E.; McCormick, A.; Cussler, E.L. Performance of a Small-Scale Haber Process. Ind. Eng. Chem. Res. 2016, 55, 3742–3750. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Chen, P. Recent progress towards mild-condition ammonia synthesis. J. Energy Chem. 2019, 36, 25–36. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Van der Ham, A.G.J.; Lefferts, L. Beyond Haber-Bosch: The renaissance of the Claude process. Int. J. Hydrog. Energy 2021, 46, 21566–21579. [Google Scholar] [CrossRef]
- Yan, H.; Gao, W.; Wang, Q.; Guan, Y.; Feng, S.; Wu, H.; Guo, Q.; Cao, H.; Guo, J.; Chen, P. Lithium Palladium Hydride Promotes Chemical Looping Ammonia Synthesis Mediated by Lithium Imide and Hydride. J. Phys. Chem. C 2021, 125, 6716–6722. [Google Scholar] [CrossRef]
- Moon, J.; Cheng, Y.; Daemen, L.; Novak, E.; Ramirez-Cuesta, A.J.; Wu, Z. On the Structural Transformation of Ni/BaH2 During a N2-H2 Chemical Looping Process for Ammonia Synthesis: A Joint In Situ Inelastic Neutron Scattering and First-Principles Simulation Study. Top. Catal. 2021, 64, 685–692. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Cai, J.; Cai, H.; Ni, J.; Lin, B.; Lin, J.; Wang, X.; Zheng, L.; Au, C.T.; et al. Operando spectroscopic and isotopic-label-directed observation of LaN-promoted Ru/ZrH2 catalyst for ammonia synthesis via associative and chemical looping route. J. Catal. 2020, 389, 218–228. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Shi, R.; Wang, B.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; Zhang, T. Tuning Oxygen Vacancies in Ultrathin TiO2 Nanosheets to Boost Photocatalytic Nitrogen Fixation up to 700 nm. Adv. Mater. 2019, 31, 1806482. [Google Scholar] [CrossRef]
- Medford, A.J.; Hatzell, M.C. Photon-Driven Nitrogen Fixation: Current Progress, Thermodynamic Considerations, and Future Outlook. ACS Catal. 2017, 7, 2624–2643. [Google Scholar] [CrossRef]
- Wang, S.; Yu, W.; Xu, S.; Han, K.; Wang, F. Ammonia from Photothermal N2Hydrogenation over Ni/TiO2Catalysts under Mild Conditions. ACS Sustain. Chem. Eng. 2022, 10, 115–123. [Google Scholar] [CrossRef]
- Hong, J.; Prawer, S.; Murphy, A.B. Plasma Catalysis as an Alternative Route for Ammonia Production: Status, Mechanisms, and Prospects for Progress. ACS Sustain. Chem. Eng. 2018, 6, 15–31. [Google Scholar] [CrossRef]
- Carreon, M.L. Plasma catalytic ammonia synthesis: State of the art and future directions. J. Phys. D Appl. Phys. 2019, 52, 483001. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Priest, C.; Li, S.; Xu, P.; Wu, G. Advanced Electrocatalysis for Energy and Environmental Sustainability via Water and Nitrogen Reactions. Adv. Mater. 2021, 33, 2000381. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Singh, A.R.; Schwalbe, J.A.; Kibsgaard, J.; Lin, J.C.; Cargnello, M.; Jaramillo, T.F.; Nørskov, J.K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10, 1621–1630. [Google Scholar] [CrossRef]
- Reichle, S.; Felderhoff, M.; Schüth, F. Mechanocatalytic Room-Temperature Synthesis of Ammonia from Its Elements Down to Atmospheric Pressure. Angew. Chem.—Int. Ed. 2021, 60, 26385–26389. [Google Scholar] [CrossRef]
- Chang, F.; Gao, W.; Guo, J.; Chen, P. Emerging Materials and Methods toward Ammonia-Based Energy Storage and Conversion. Adv. Mater. 2021, 33, 2005721. [Google Scholar] [CrossRef]
- Gao, W.; Guo, J.; Chen, P. Hydrides, Amides and Imides Mediated Ammonia Synthesis and Decomposition. Chin. J. Chem. 2019, 37, 442–451. [Google Scholar] [CrossRef]
- Marakatti, V.S.; Gaigneaux, E.M. Recent Advances in Heterogeneous Catalysis for Ammonia Synthesis. ChemCatChem 2020, 12, 5838–5857. [Google Scholar] [CrossRef]
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Saadatjou, N.; Jafari, A.; Sahebdelfar, S. Ruthenium Nanocatalysts for Ammonia Synthesis: A Review. Chem. Eng. Commun. 2015, 202, 420–448. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Zhou, Y.; Wang, X.; Au, C.-T.; Jiang, L. Review on catalytic roles of rare earth elements in ammonia synthesis: Development and perspective. J. Rare Earths 2022, 40, 1–10. [Google Scholar] [CrossRef]
- Nakaya, Y.; Furukawa, S. Catalysis of Alloys: Classification, Principles, and Design for a Variety of Materials and Reactions. Chem. Rev. 2022, 123, 5859–5947. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, P. Interplay of Alkali, Transition Metals, Nitrogen, and Hydrogen in Ammonia Synthesis and Decomposition Reactions. Acc. Chem. Res. 2021, 54, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.W.; Hara, M.; Hosono, H. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef]
- Ojelade, O.A.; Zaman, S.F. Ammonia decomposition for hydrogen production: A thermodynamic study. Chem. Pap. 2021, 75, 57–65. [Google Scholar] [CrossRef]
- Ertl, G. Surface Science and Catalysis—Studies on the Mechanism of Ammonia Synthesis: The P. H. Emmett Award Address. Catal. Rev. 1980, 21, 201–223. [Google Scholar] [CrossRef]
- Almquist, J.A.; Crittenden, E.D. A Study of Pure-Iron and Promoted-iron Catalysts for Ammonia Synthesis. Ind. Eng. Chem. 1926, 18, 1307–1309. [Google Scholar] [CrossRef]
- Aika, K.-I. Role of alkali promoter in ammonia synthesis over ruthenium catalysts—Effect on reaction mechanism. Catal. Today 2017, 286, 14–20. [Google Scholar] [CrossRef]
- García-García, F.R.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Role of B5-Type Sites in Ru Catalysts used for the NH3 Decomposition Reaction. Top. Catal. 2009, 52, 758–764. [Google Scholar] [CrossRef]
- van Hardeveld, R.; van Montfoort, A. The influence of crystallite size on the adsorption of molecular nitrogen on nickel, palladium and platinum: An infrared and electron-microscopic study. Surf. Sci. 1966, 4, 396–430. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Szmigiel, D.; Kowalczyk, Z.; Jodzis, S.; Zielinski, J. Ammonia decomposition over the carbon-based ruthenium catalyst promoted with barium or cesium. J. Catal. 2003, 218, 465–469. [Google Scholar] [CrossRef]
- Osozawa, M.; Hori, A.; Fukai, K.; Honma, T.; Oshima, K.; Satokawa, S. Improvement in ammonia synthesis activity on ruthenium catalyst using ceria support modified a large amount of cesium promoter. Int. J. Hydrog. Energy 2022, 47, 2433–2441. [Google Scholar] [CrossRef]
- Zhang, Z.; Karakaya, C.; Kee, R.J.; Way, J.D.; Wolden, C.A. Barium-Promoted Ruthenium Catalysts on Yittria-Stabilized Zirconia Supports for Ammonia Synthesis. ACS Sustain. Chem. Eng. 2019, 7, 18038–18047. [Google Scholar] [CrossRef]
- Nishi, M.; Chen, S.Y.; Takagi, H. Mild ammonia synthesis over Ba-promoted Ru/MPC catalysts: Effects of the Ba/Ru ratio and the mesoporous structure. Catalysts 2019, 9, 480. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Sasase, M.; Kishida, K.; Kobayashi, Y.; Nishiyama, K.; Tada, T.; Kawamura, S.; Yokoyama, T.; Hara, M.; et al. Self-organized Ruthenium-Barium Core-Shell Nanoparticles on a Mesoporous Calcium Amide Matrix for Efficient Low-Temperature Ammonia Synthesis. Angew. Chem. 2018, 130, 2678–2682. [Google Scholar] [CrossRef]
- Guraya, M.; Sprenger, S.; Rarog-Pilecka, W.; Szmigiel, D.; Kowalczyk, Z.; Muhler, M. The effect of promoters on the electronic structure of ruthenium catalysts supported on carbon. Appl. Surf. Sci. 2004, 238, 77–81. [Google Scholar] [CrossRef]
- Raróg-Pilecka, W.; Miśkiewicz, E.; Szmigiel, D.; Kowalczyk, Z. Structure sensitivity of ammonia synthesis over promoted ruthenium catalysts supported on graphitised carbon. J. Catal. 2005, 231, 11–19. [Google Scholar] [CrossRef]
- Aika, K.-I.; Hori, H.; Ozaki, A. Activation of nitrogen by alkali metal promoted transition metal I. Ammonia synthesis over ruthenium promoted by alkali metal. J. Catal. 1972, 27, 424–431. [Google Scholar] [CrossRef]
- Seetharamulu, P.; Hari Prasad Reddy, K.; Padmasri, A.H.; Rama Rao, K.S.; David Raju, B. Role of promoters on highly active nano-Ru catalyst supported on Mg–Al hydrotalcite precursor for the synthesis of ammonia. Catal. Today 2009, 141, 94–98. [Google Scholar] [CrossRef]
- Wang, Y.; Wildfire, C.; Khan, T.S.; Shekhawat, D.; Hu, J.; Tavadze, P.; Quiñones-Fernández, R.; Moreno, S. Effects of support and promoter on Ru catalyst activity in microwave-assisted ammonia synthesis. Chem. Eng. J. 2021, 425, 130546. [Google Scholar] [CrossRef]
- Miyahara, S.I.; Sato, K.; Kawano, Y.; Imamura, K.; Ogura, Y.; Tsujimaru, K.; Nagaoka, K. Ammonia synthesis over lanthanoid oxide–supported ruthenium catalysts. Catal. Today 2021, 376, 36–40. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Y.; Heng, L.; Wang, X.; Ni, J.; Lin, J.; Jiang, L. Morphology Effect of Ceria on the Catalytic Performances of Ru/CeO2 Catalysts for Ammonia Synthesis. Ind. Eng. Chem. Res. 2018, 57, 9127–9135. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, J.; Ni, J.; Wang, R.; Wei, K. Highly efficient Ru/Sm2O3-CeO2 catalyst for ammonia synthesis. Catal. Commun. 2011, 15, 23–26. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient visible light nitrogen fixation with BiOBr nanosheets of oxygen vacancies on the exposed {001} Facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar] [CrossRef]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic Conversion of Nitrogen to Ammonia with Water on Surface Oxygen Vacancies of Titanium Dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Zhao, Z.J.; Yang, W.; Li, J.F.; Li, A.; Yang, Z.; Ozin, G.A.; Gong, J. Promoted Fixation of Molecular Nitrogen with Surface Oxygen Vacancies on Plasmon-Enhanced TiO2 Photoelectrodes. Angew. Chem. Int. Ed. 2018, 57, 5278–5282. [Google Scholar] [CrossRef]
- Dye, J.L. Electrons as anions. Science 2003, 301, 607–608. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Ishikawa, H.; Yamagata, K.; Nakao, T.; Tada, T.; Matsuishi, S.; Yokoyama, T.; Hara, M.; Hosono, H. Essential role of hydride ion in ruthenium-based ammonia synthesis catalysts. Chem. Sci. 2016, 7, 4036–4043. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Ye, T.N.; Kobayashi, Y.; Sasase, M.; Kitano, M.; Hosono, H. Synthesis of rare-earth-based metallic electride nanoparticles and their catalytic applications to selective hydrogenation and ammonia synthesis. ACS Catal. 2018, 8, 11054–11058. [Google Scholar] [CrossRef]
- Wu, J.; Gong, Y.; Inoshita, T.; Fredrickson, D.C.; Wang, J.; Lu, Y.; Kitano, M.; Hosono, H. Tiered Electron Anions in Multiple Voids of LaScSi and Their Applications to Ammonia Synthesis. Adv. Mater. 2017, 29, 1700924. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitano, M.; Kim, S.W.; Yokoyama, T.; Hara, M.; Hosono, H. Highly dispersed ru on electride [Ca24Al28O64]4+(e-)4 as a catalyst for ammonia synthesis. ACS Catal. 2014, 4, 674–680. [Google Scholar] [CrossRef]
- Kitano, M.; Kanbara, S.; Inoue, Y.; Kuganathan, N.; Sushko, P.V.; Yokoyama, T.; Hara, M.; Hosono, H. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat. Commun. 2015, 6, 6371. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.N.; Park, S.W.; Lu, Y.; Li, J.; Sasase, M.; Kitano, M.; Tada, T.; Hosono, H. Vacancy-enabled N2 activation for ammonia synthesis on an Ni-loaded catalyst. Nature 2020, 583, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.N.; Park, S.W.; Lu, Y.; Li, J.; Sasase, M.; Kitano, M.; Hosono, H. Contribution of Nitrogen Vacancies to Ammonia Synthesis over Metal Nitride Catalysts. J. Am. Chem. Soc. 2020, 142, 14374–14383. [Google Scholar] [CrossRef]
- Chang, F.; Guan, Y.; Chang, X.; Guo, J.; Wang, P.; Gao, W.; Wu, G.; Zheng, J.; Li, X.; Chen, P. Alkali and Alkaline Earth Hydrides-Driven N2 Activation and Transformation over Mn Nitride Catalyst. J. Am. Chem. Soc. 2018, 140, 14799–14806. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitano, M.; Kishida, K.; Abe, H.; Niwa, Y.; Sasase, M.; Fujita, Y.; Ishikawa, H.; Yokoyama, T.; Hara, M.; et al. Efficient and Stable Ammonia Synthesis by Self-Organized Flat Ru Nanoparticles on Calcium Amide. ACS Catal. 2016, 6, 7577–7584. [Google Scholar] [CrossRef]
- Wang, P.; Chang, F.; Gao, W.; Guo, J.; Wu, G.; He, T.; Chen, P. Breaking scaling relations to achieve low-temperature ammonia synthesis through LiH-mediated nitrogen transfer and hydrogenation. Nat. Chem. 2017, 9, 64–70. [Google Scholar] [CrossRef]
- Hattori, M.; Iijima, S.; Nakao, T.; Hosono, H.; Hara, M. Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C. Nat. Commun. 2020, 11, 2001. [Google Scholar] [CrossRef]
- Croisé, C.; Alabd, K.; Tencé, S.; Gaudin, E.; Villesuzanne, A.; Courtois, X.; Bion, N.; Can, F. Influence of the Rare Earth (R) Element in Ru-supported RScSi Electride-like Intermetallic Catalysts for Ammonia Synthesis at Low Pressure: Insight into NH3 Formation Mechanism. ChemCatChem 2023, 15, e202201172. [Google Scholar] [CrossRef]
- Croisé, C.; Alabd, K.; Villesuzanne, A.; Can, F.; Courtois, X.; Gaudin, E.; Tencé, S.; Bion, N. Role of hydride ion within Ru/LaScSi and Ru/CeTiGe catalysts for NH3 synthesis: A combination of DFT and experimental nitrogen isotopic exchange studies. Catal. Commun. 2023, 179, 106689. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Li, C.; Bao, X.; Hosono, H.; Wang, J. Insight into rare-earth-incorporated catalysts: The chance for a more efficient ammonia synthesis. J. Adv. Ceram. 2022, 11, 1499–1529. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Wu, J.; Song, X.; Yang, X.; Bao, X.; Han, X.; Kitano, M.; Wang, J.; Hosono, H. Unique Catalytic Mechanism for Ru-Loaded Ternary Intermetallic Electrides for Ammonia Synthesis. J. Am. Chem. Soc. 2022, 144, 8683–8692. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Gong, Y.; Kitano, M.; Inoshita, T.; Hosono, H. Intermetallic Electride Catalyst as a Platform for Ammonia Synthesis. Angew. Chem. Int. Ed. 2019, 58, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.; Wang, H.; Lu, Y.; Ye, T.; Sasase, M.; Wu, X.; Kitano, M.; Inoshita, T.; Hosono, H. Acid-durable electride with layered ruthenium for ammonia synthesis: Boosting the activity via selective etching. Chem. Sci. 2019, 10, 5712–5718. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Liu, J.C.; Xu, C.Q.; Li, J.; Xiao, H. Breaking the scaling relations for efficient N2-to-NH3 conversion by a bowl active site design: Insight from LaRuSi and isostructural electrides. Chin. J. Catal. 2022, 43, 2183–2192. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Dahl, S.; Clausen, B.G.S.; Bahn, S.; Logadottir, A.; Nørskov, J.K. Catalyst design by interpolation in the periodic table: Bimetallic ammonia synthesis catalysts. J. Am. Chem. Soc. 2001, 123, 8404–8405. [Google Scholar] [CrossRef]
- Sato, K.; Nagaoka, K. Boosting Ammonia Synthesis under Mild Reaction Conditions by Precise Control of the Basic Oxide–Ru Interface. Chem. Lett. 2021, 50, 687–696. [Google Scholar] [CrossRef]
- Yan, H.; Gao, W.; Cui, J.; Zhang, W.; Pei, Q.; Wang, Q.; Guan, Y.; Feng, S.; Wu, H.; Cao, H.; et al. Dinitrogen fixation mediated by lanthanum hydride. J. Energy Chem. 2022, 72, 1–7. [Google Scholar] [CrossRef]
- Ooya, K.; Li, J.; Fukui, K.; Iimura, S.; Nakao, T.; Ogasawara, K.; Sasase, M.; Abe, H.; Niwa, Y.; Kitano, M.; et al. Ruthenium Catalysts Promoted by Lanthanide Oxyhydrides with High Hydride-Ion Mobility for Low-Temperature Ammonia Synthesis. Adv. Energy Mater. 2021, 11, 2003723. [Google Scholar] [CrossRef]
- Fukui, K.; Iimura, S.; Iskandarov, A.; Tada, T.; Hosono, H. Room-Temperature Fast H-Conduction in Oxygen-Substituted Lanthanum Hydride. J. Am. Chem. Soc. 2022, 144, 1523–1527. [Google Scholar] [CrossRef]
- Ye, T.N.; Lu, Y.; Kobayashi, Y.; Li, J.; Park, S.W.; Sasase, M.; Kitano, M.; Hosono, H. Efficient Ammonia Synthesis over Phase-Separated Nickel-Based Intermetallic Catalysts. J. Phys. Chem. C 2020, 124, 28589–28595. [Google Scholar] [CrossRef]
- Miyahara, S.I.; Sato, K.; Tsujimaru, K.; Wada, Y.; Ogura, Y.; Toriyama, T.; Yamamoto, T.; Matsumura, S.; Inazu, K.; Nagaoka, K. Co Nanoparticle Catalysts Encapsulated by BaO-La2O3Nanofractions for Efficient Ammonia Synthesis Under Mild Reaction Conditions. ACS Omega 2022, 7, 24452–24460. [Google Scholar] [CrossRef] [PubMed]
- Che, M. Nobel Prize in chemistry 1912 to Sabatier: Organic chemistry or catalysis? Catal. Today 2013, 218–219, 162–171. [Google Scholar] [CrossRef]
- Vojvodic, A.; Medford, A.J.; Studt, F.; Abild-Pedersen, F.; Khan, T.S.; Bligaard, T.; Nørskov, J.K. Exploring the limits: A low-pressure, low-temperature Haber-Bosch process. Chem. Phys. Lett. 2014, 598, 108–112. [Google Scholar] [CrossRef]
- Nakao, T.; Tada, T.; Hosono, H. First-Principles and Microkinetic Study on the Mechanism for Ammonia Synthesis Using Ru-Loaded Hydride Catalyst. J. Phys. Chem. C 2020, 124, 2070–2078. [Google Scholar] [CrossRef]
- Kitano, M.; Kujirai, J.; Ogasawara, K.; Matsuishi, S.; Tada, T.; Abe, H.; Niwa, Y.; Hosono, H. Low-Temperature Synthesis of Perovskite Oxynitride-Hydrides as Ammonia Synthesis Catalysts. J. Am. Chem. Soc. 2019, 141, 20344–20353. [Google Scholar] [CrossRef]
- Hosono, H.; Kitano, M. Advances in Materials and Applications of Inorganic Electrides. Chem. Rev. 2021, 121, 3121–3185. [Google Scholar] [CrossRef]
- Tang, Y.; Kobayashi, Y.; Masuda, N.; Uchida, Y.; Okamoto, H.; Kageyama, T.; Hosokawa, S.; Loyer, F.; Mitsuhara, K.; Yamanaka, K.; et al. Metal-Dependent Support Effects of Oxyhydride-Supported Ru, Fe, Co Catalysts for Ammonia Synthesis. Adv. Energy Mater. 2018, 8, 1801772. [Google Scholar] [CrossRef]
- Gao, W.; Feng, S.; Yan, H.; Wang, Q.; Xie, H.; Jiang, L.; Zhang, W.; Guan, Y.; Wu, H.; Cao, H.; et al. In situ formed Co from a Co-Mg-O solid solution synergizing with LiH for efficient ammonia synthesis. Chem. Commun. 2021, 57, 8576–8579. [Google Scholar] [CrossRef]
- Hu, Z.; Mahin, J.; Datta, S.; Bell, T.E.; Torrente-Murciano, L. Ru-Based Catalysts for H2 Production from Ammonia: Effect of 1D Support. Top. Catal. 2019, 62, 1169–1177. [Google Scholar] [CrossRef]
- Zhu, L.; Cadigan, C.; Duan, C.; Huang, J.; Bian, L.; Le, L.; Hernandez, C.H.; Avance, V.; O’Hayre, R.; Sullivan, N.P. Ammonia-fed reversible protonic ceramic fuel cells with Ru-based catalyst. Commun. Chem. 2021, 4, 121. [Google Scholar] [CrossRef] [PubMed]
- López-Rodríguez, S.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; Bueno-López, A. Effect of Ru loading on Ru/CeO2 catalysts for CO2 methanation. Mol. Catal. 2021, 515, 111911. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kitano, M.; Kawamura, S.; Yokoyama, T.; Hosono, H. Kinetic evidence: The rate-determining step for ammonia synthesis over electride-supported Ru catalysts is no longer the nitrogen dissociation step. Catal. Sci. Technol. 2017, 7, 47–50. [Google Scholar] [CrossRef]
- Hosono, H. Electron Transfer from Support/Promotor to Metal Catalyst: Requirements for Effective Support. Catal. Lett. 2022, 152, 307–314. [Google Scholar] [CrossRef]
- Kitano, M.; Yamagata, K.; Hosono, H. Why Ca2NH works as an efficient and stable support of Ru catalyst in ammonia synthesis. Res. Chem. Intermed. 2021, 47, 235–248. [Google Scholar] [CrossRef]
- Kishida, K.; Kitano, M.; Sasase, M.; Sushko, P.V.; Abe, H.; Niwa, Y.; Ogasawara, K.; Yokoyama, T.; Hosono, H. Air-Stable Calcium Cyanamide-Supported Ruthenium Catalyst for Ammonia Synthesis and Decomposition. ACS Appl. Energy Mater. 2020, 3, 6573–6582. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Okunaka, M.; Kitano, M.; Matsuishi, S.; Yokoyama, T.; Hosono, H. Hydride-Based Electride Material, LnH2 (Ln = La, Ce, or Y). Inorg. Chem. 2016, 55, 8833–8838. [Google Scholar] [CrossRef]
- Sato, K.; Imamura, K.; Kawano, Y.; Miyahara, S.I.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem. Sci. 2016, 8, 674–679. [Google Scholar] [CrossRef]
- Hargreaves, J.S.J. Nitrides as ammonia synthesis catalysts and as potential nitrogen transfer reagents. Appl. Petrochem. Res. 2014, 4, 3–10. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Kitano, M.; Wang, J.; Ye, T.N.; Li, J.; Kobayashi, Y.; Kishida, K.; Abe, H.; Niwa, Y.; et al. Ternary intermetallic LaCoSi as a catalyst for N2 activation. Nat. Catal. 2018, 1, 178–185. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyo-Caire, J.; Diaz-Perez, M.A.; Lara-Angulo, M.A.; Serrano-Ruiz, J.C. A Conceptual Approach for the Design of New Catalysts for Ammonia Synthesis: A Metal—Support Interactions Review. Nanomaterials 2023, 13, 2914. https://doi.org/10.3390/nano13222914
Arroyo-Caire J, Diaz-Perez MA, Lara-Angulo MA, Serrano-Ruiz JC. A Conceptual Approach for the Design of New Catalysts for Ammonia Synthesis: A Metal—Support Interactions Review. Nanomaterials. 2023; 13(22):2914. https://doi.org/10.3390/nano13222914
Chicago/Turabian StyleArroyo-Caire, Javier, Manuel Antonio Diaz-Perez, Mayra Anabel Lara-Angulo, and Juan Carlos Serrano-Ruiz. 2023. "A Conceptual Approach for the Design of New Catalysts for Ammonia Synthesis: A Metal—Support Interactions Review" Nanomaterials 13, no. 22: 2914. https://doi.org/10.3390/nano13222914