Effect of Europium Substitution on the Structural, Magnetic and Relaxivity Properties of Mn-Zn Ferrite Nanoparticles: A Dual-Mode MRI Contrast-Agent Candidate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coprecipitation Synthesis of Mn0.6Zn0.4EuxFe2−xO4
2.3. Hydrothermal Synthesis of Mn0.6Zn0.4EuxFe2−xO4
2.4. PF-127 Coating
2.5. X-ray Diffraction
2.6. Transmission Electron Microscopy
2.7. Magnetic Characterization
2.7.1. Vibrating Sample Magnetometer
2.7.2. Curie Temperature
2.8. Fourier-Transform Infrared Spectroscopy
2.9. Elemental Analysis
2.10. Colloidal Stability
2.11. Relaxometery Measurements
3. Results and Discussion
3.1. Structural Characterization
3.1.1. X-ray Diffraction Study
3.1.2. TEM Analysis
3.1.3. Fourier-Transform Infrared Spectra
3.2. Magnetic Properties
3.3. Colloidal Stability
3.4. Relaxometery Measurements
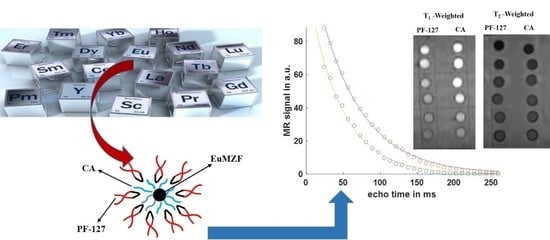
3.4.1. Effect of Surface Design on Relaxivities
3.4.2. Effect of Eu Substitution on Relaxivities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sai Ram, B.; Paul, A.K.; Kulkarni, S.V. Soft Magnetic Materials and Their Applications in Transformers. J. Magn. Magn. Mater. 2021, 537, 168210. [Google Scholar] [CrossRef]
- Praveena, K.; Sadhana, K.; Bharadwaj, S.; Murthy, S.R. Development of Nanocrystalline Mn–Zn Ferrites for High Frequency Transformer Applications. J. Magn. Magn. Mater. 2009, 321, 2433–2437. [Google Scholar] [CrossRef]
- Kurimský, J.; Rajňák, M.; Cimbala, R.; Rajnič, J.; Timko, M.; Kopčanský, P. Effect of Magnetic Nanoparticles on Partial Discharges in Transformer Oil. J. Magn. Magn. Mater. 2020, 496, 165923. [Google Scholar] [CrossRef]
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the Application of Magnetic Nanoparticles for Sensing. Adv. Mater. Deerfield Beach Fla 2019, 31, e1904385. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.A.P. Sensors and Biosensors Based on Magnetic Nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Kumar, A.; Almomani, T. Heavy Metal Ions Removal from Industrial Wastewater Using Magnetic Nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Masjedi, A.; Askarizadeh, E.; Baniyaghoob, S. Magnetic Nanoparticles Surface-Modified with Tridentate Ligands for Removal of Heavy Metal Ions from Water. Mater. Chem. Phys. 2020, 249, 122917. [Google Scholar] [CrossRef]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 188. [Google Scholar] [CrossRef] [Green Version]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of Magnetic Nanoparticles in Biomedicine. J. Phys. Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. In Surface-modified Nanobiomaterials for Electrochemical and Biomedicine Applications; Puente-Santiago, A.R., Rodríguez-Padrón, D., Eds.; Topics in Current Chemistry Collections; Springer International Publishing: Cham, Switzerland, 2020; pp. 49–91. ISBN 978-3-030-55502-3. [Google Scholar]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic Nanoparticles with Dual Functional Properties: Drug Delivery and Magnetic Resonance Imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef]
- Thiesen, B.; Jordan, A. Clinical Applications of Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperthermia 2008, 24, 467–474. [Google Scholar] [CrossRef]
- Alonso, J.; Barandiarán, J.M.; Fernández Barquín, L.; García-Arribas, A. Chapter 1—Magnetic Nanoparticles, Synthesis, Properties, and Applications. In Magnetic Nanostructured Materials; El-Gendy, A.A., Barandiarán, J.M., Hadimani, R.L., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. ISBN 978-0-12-813904-2. [Google Scholar]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Szczygieł, I.; Winiarska, K.; Sobianowska-Turek, A. The Study of Thermal, Microstructural and Magnetic Properties of Manganese–Zinc Ferrite Prepared by Co-Precipitation Method Using Different Precipitants. J. Therm. Anal. Calorim. 2018, 134. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yan, C.; Xie, J.; Yan, D.; Hu, K.; Huang, S.; Liu, J.; Zhang, Y.; Gu, N.; Xiong, F. High-Performance Worm-like Mn–Zn Ferrite Theranostic Nanoagents and the Application on Tumor Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 29536–29548. [Google Scholar] [CrossRef]
- Maksoud, M.I.A.A.; Ghobashy, M.M.; Kodous, A.S.; Fahim, R.A.; Osman, A.I.; Al-Muhtaseb, A.H.; Rooney, D.W.; Mamdouh, M.A.; Nady, N.; Ashour, A.H. Insights on Magnetic Spinel Ferrites for Targeted Drug Delivery and Hyperthermia Applications. Nanotechnol. Rev. 2022, 11, 372–413. [Google Scholar] [CrossRef]
- Lin, M.; Huang, J.; Sha, M. Recent Advances in Nanosized Mn-Zn Ferrite Magnetic Fluid Hyperthermia for Cancer Treatment. J. Nanosci. Nanotechnol. 2014, 14, 792–802. [Google Scholar] [CrossRef]
- Hu, P.; Yang, H.; Pan, D.; Wang, H.; Tian, J.; Zhang, S.; Wang, X.; Volinsky, A.A. Heat Treatment Effects on Microstructure and Magnetic Properties of Mn–Zn Ferrite Powders. J. Magn. Magn. Mater. 2010, 322, 173–177. [Google Scholar] [CrossRef]
- Salehpour, F.; Khorramdin, A.; Shokrollahi, H.; Pezeshki, A.; Mirzaei, F.; Nader, N.D. Synthesis of Zn-Doped Manganese Ferrite Nanoparticles Via Coprecipitation Method for Magnetic Resonance Imaging Contrast Agent. J. Nanotechnol. Eng. Med. 2014, 5. [Google Scholar] [CrossRef]
- Zheng, R.; Guo, J.; Cai, X.; Bin, L.; Lu, C.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Manganese Complexes and Manganese-Based Metal-Organic Frameworks as Contrast Agents in MRI and Chemotherapeutics Agents: Applications and Prospects. Colloids Surf. B Biointerfaces 2022, 213, 112432. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent Advances in Ti-Based MOFs in Biomedical Applications. Dalton Trans. 2022, 51, 14817–14832. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface Impact on Nanoparticle-Based Magnetic Resonance Imaging Contrast Agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Lee, G.T.; Kim, H.-K.; Sung, B.; Lee, Y.; Kim, M.; Chang, Y.; Seo, J.H. Surface Design of Eu-Doped Iron Oxide Nanoparticles for Tuning the Magnetic Relaxivity. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in Magnetic Resonance Imaging: From Simple to Dual Contrast Agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Wang, Z.; Xiang, H.; Xu, X.; Zou, J.; Lu, C. Biocompatible Superparamagnetic Europium-Doped Iron Oxide Nanoparticle Clusters as Multifunctional Nanoprobes for Multimodal In Vivo Imaging. ACS Appl. Mater. Interfaces 2021, 13, 33850–33861. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.; Jativa, S.D.; Kaittanis, C.; Normand, G.; Grimm, J.; Perez, J.M. Gadolinium-Encapsulating Iron Oxide Nanoprobe as Activatable NMR/MRI Contrast Agent. ACS Nano 2012, 6, 7281–7294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.; Bae, H.; Iqbal, Y.; Rhee, I.; Hong, S.; Chang, Y.; Lee, J.; Sohn, D. Chitosan-Coated Nickel-Ferrite Nanoparticles as Contrast Agents in Magnetic Resonance Imaging. J. Magn. Magn. Mater. 2015, 381, 151–157. [Google Scholar] [CrossRef]
- Xiao, R.; Ding, J.; Chen, J.; Zhao, Z.; He, L.; Wang, H.; Huang, S.; Luo, B. Citric Acid Coated Ultrasmall Superparamagnetic Iron Oxide Nanoparticles Conjugated with Lactoferrin for Targeted Negative MR Imaging of Glioma. J. Biomater. Appl. 2021, 36, 15–25. [Google Scholar] [CrossRef]
- Faraji, S.; Dini, G.; Zahraei, M. Polyethylene Glycol-Coated Manganese-Ferrite Nanoparticles as Contrast Agents for Magnetic Resonance Imaging. J. Magn. Magn. Mater. 2019, 475, 137–145. [Google Scholar] [CrossRef]
- Unterweger, H.; Dézsi, L.; Matuszak, J.; Janko, C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; et al. Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles for Magnetic Resonance Imaging: Evaluation of Size-Dependent Imaging Properties, Storage Stability and Safety. Int. J. Nanomed. 2018, 13, 1899–1915. [Google Scholar] [CrossRef] [Green Version]
- Kostevšek, N.; Cheung, C.C.L.; Serša, I.; Kreft, M.E.; Monaco, I.; Comes Franchini, M.; Vidmar, J.; Al-Jamal, W.T. Magneto-Liposomes as MRI Contrast Agents: A Systematic Study of Different Liposomal Formulations. Nanomaterials 2020, 10, 889. [Google Scholar] [CrossRef]
- Shatooti, S.; Mozaffari, M.; Reiter, G.; Zahn, D.; Dutz, S. Heat Dissipation in Sm3+ and Zn2+ Co-Substituted Magnetite (Zn0.1SmxFe2.9-XO4) Nanoparticles Coated with Citric Acid and Pluronic F127 for Hyperthermia Application. Sci. Rep. 2021, 11, 16795. [Google Scholar] [CrossRef]
- Vu-Quang, H.; Vinding, M.S.; Nielsen, T.; Ullisch, M.G.; Nielsen, N.C.; Nguyen, D.-T.; Kjems, J. Pluronic F127-Folate Coated Super Paramagenic Iron Oxide Nanoparticles as Contrast Agent for Cancer Diagnosis in Magnetic Resonance Imaging. Polymers 2019, 11, 743. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Yao, D.; Yin, G.; Huang, Z.; Pu, X. Peptide-Decorated Ultrasmall Superparamagnetic Nanoparticles as Active Targeting MRI Contrast Agents for Ovarian Tumors. ACS Appl. Mater. Interfaces 2019, 11, 41038–41050. [Google Scholar] [CrossRef]
- Sang, S.; Gu, Y.; Huang, K. Effect of Additive on Synthesis of MnZn Ferrite Nanocrystal by Hydrothermal Crystallization. J. Cent. South Univ. Technol. 2003, 10, 38–43. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Beregi, E.; Hild, E.; Sztaniszláv, A.; Rudnay, G.; Sztatisz, J. Investigation of Solid State Reaction in 3Y2O3:5Fe2O3 by DTG(M), X-ray and IR Spectroscopic Methods. J. Magn. Magn. Mater. 1984, 41, 73–74. [Google Scholar] [CrossRef]
- Bock, M.; Schulz, J.; Ueltzhoeffer, S.; Giesel, F.; Voth, M.; Essig, M. Intravascular Contrast Agent T1 Shortening: Fast T1 Relaxometry in a Carotid Volunteer Study. Magn. Reson. Mater. Phys. Biol. Med. 2008, 21, 363–368. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Rozman, M.; Drofenik, M. Hydrothermal Synthesis of Manganese Zinc Ferrites. J. Am. Ceram. Soc. 1995, 78, 2449–2455. [Google Scholar] [CrossRef]
- Zahraei, M.; Monshi, A.; Morales, M.d.P.; Shahbazi-Gahrouei, D.; Amirnasr, M.; Behdadfar, B. Hydrothermal Synthesis of Fine Stabilized Superparamagnetic Nanoparticles of Zn2+ Substituted Manganese Ferrite. J. Magn. Magn. Mater. 2015, 393, 429–436. [Google Scholar] [CrossRef]
- Li, X.; Sun, R.; Luo, B.; Zhang, A.; Xia, A.; Jin, C. Synthesis and Magnetic Properties of Manganese–Zinc Ferrite Nanoparticles Obtained via a Hydrothermal Method. J. Mater. Sci. Mater. Electron. 2017, 28. [Google Scholar] [CrossRef]
- Rezaei, B.; Kermanpur, A.; Labbaf, S. Effect of Mn Addition on the Structural and Magnetic Properties of Zn-Ferrite Nanoparticles. J. Magn. Magn. Mater. 2019, 481, 16–24. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A Microporous 2D Cobalt-Based MOF with Pyridyl Sites and Open Metal Sites for Selective Adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; IEEE/Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0-471-47741-9. [Google Scholar]
- Stoner, E.C.; Wohlfarth, E.P. A Mechanism of Magnetic Hysteresis in Heterogeneous Alloys. Philos. Trans. R. Soc. Lond. Ser. Math. Phys. Sci. 1948, 240, 599–642. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Mane, M.L.; Yasukawa, Y.; Liu, X.; Morisako, A. Self-Ignited High Temperature Synthesis and Enhanced Super-Exchange Interactions of Ho3+–Mn2+–Fe3+–O2− Ferromagnetic Nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 2347–2357. [Google Scholar] [CrossRef]
- Néel, L. Antiferromagnetism and Ferrimagnetism. Proc. Phys. Soc. Sect. A 1952, 65, 869. [Google Scholar] [CrossRef]
- Hashim, M.; Shirsath, S.; Meena, S.; Mane, M.; Kumar, S.; Bhatt, P.; Kumar, R.; Prasad, N.; Alla, S.K.; Shah, J.; et al. Manganese Ferrite Prepared Using Reverse Micelle Process: Structural and Magnetic Properties Characterization. J. Alloys Compd. 2015, 642, 70–77. [Google Scholar] [CrossRef]
- Mohamed, W.S.; Abu-Dief, A.M. Impact of Rare Earth Europium (RE-Eu3+) Ions Substitution on Microstructural, Optical and Magnetic Properties of CoFe2−xEuxO4 Nanosystems. Ceram. Int. 2020, 46, 16196–16209. [Google Scholar] [CrossRef]
- Dutz, S.; Buske, N.; Landers, J.; Gräfe, C.; Wende, H.; Clement, J.H. Biocompatible Magnetic Fluids of Co-Doped Iron Oxide Nanoparticles with Tunable Magnetic Properties. Nanomaterials 2020, 10, 1019. [Google Scholar] [CrossRef]
- Xing, Q.; Peng, Z.; Wang, C.; Fu, Z.; Fu, X. Doping Effect of Y3+ Ions on the Microstructural and Electromagnetic Properties of Mn–Zn Ferrites. Phys. B Condens. Matter 2012, 407, 388–392. [Google Scholar] [CrossRef]
- Laha, S.S.; Thorat, N.; Singh, G.; Sathish, C.; Yi, J.; Dixit, A.; Vinu, A. Rare-Earth Doped Iron Oxide Nanostructures for Cancer Theranostics: Magnetic Hyperthermia and Magnetic Resonance Imaging. Small 2021. [Google Scholar] [CrossRef]
- Sánchez, J.; Rodríguez-Reyes, M.; Hernández, D.; Ávila-Orta, C.; Reyes, P. Heating Capacity and Biocompatibility of Pluronic-Coated Manganese Gallium Ferrites for Magnetic Hyperthermia Treatment. Colloids Surf. Physicochem. Eng. Asp. 2020, 612, 125986. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. The Role of Interactions in Systems of Single Domain Ferrimagnetic Iron Oxide Nanoparticles. J. Nano- Electron. Phys. 2012, 4, 20101–20107. [Google Scholar]
- Singh, M.; Sud, S.P. Controlling the Properties of Magnesium–Manganese Ferrites. Mater. Sci. Eng. B 2001, 83, 180–184. [Google Scholar] [CrossRef]
- Angadi, V.J.; Choudhury, L.; Sadhana, K.; Liu, H.-L.; Sandhya, R.; Matteppanavar, S.; Rudraswamy, B.; Pattar, V.; Anavekar, R.V.; Praveena, K. Structural, Electrical and Magnetic Properties of Sc3+ Doped Mn-Zn Ferrite Nanoparticles. J. Magn. Magn. Mater. 2017, 424, 1–11. [Google Scholar] [CrossRef]
- Tóth, É.; Helm, L.; Merbach, A.E. Relaxivity of MRI Contrast Agents. In Contrast Agents I: Magnetic Resonance Imaging; Krause, W., Ed.; Topics in Current Chemistry; Springer: Berlin, Heidelberg, 2002; pp. 61–101. ISBN 978-3-540-45733-6. [Google Scholar]
- Laurent, S.; Henoumont, C.; Stanicki, D.; Boutry, S.; Lipani, E.; Belaid, S.; Muller, R.N.; Vander Elst, L. Magnetic Properties. In MRI Contrast Agents: From Molecules to Particles; SpringerBriefs in Applied Sciences and Technology; Springer: Singapore, 2017; pp. 5–11. ISBN 978-981-10-2529-7. [Google Scholar]
- Keasberry, N.A.; Bañobre-López, M.; Wood, C.; Stasiuk, G.J.; Gallo, J.; Long, N.J. Tuning the Relaxation Rates of Dual-Mode T1/T2 Nanoparticle Contrast Agents: A Study into the Ideal System. Nanoscale 2015, 7, 16119–16128. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Zahraei, M.; Marciello, M.; Lazaro-Carrillo, A.; Villanueva, A.; Herranz, F.; Talelli, M.; Costo, R.; Monshi, A.; Shahbazi-Gahrouei, D.; Amirnasr, M.; et al. Versatile Theranostics Agents Designed by Coating Ferrite Nanoparticles with Biocompatible Polymers. Nanotechnology 2016, 27, 255702. [Google Scholar] [CrossRef]
- Leng, J.; Li, J.; Ren, J.; Deng, L.; Lin, C. Star–Block Copolymer Micellar Nanocomposites with Mn,Zn-Doped Nano-Ferrite as Superparamagnetic MRI Contrast Agent for Tumor Imaging. Mater. Lett. 2015, 152, 185–188. [Google Scholar] [CrossRef]
- Sobhani, T.; Shahbazi-Gahrouei, D.; Rostami, M.; Zahraei, M.; Farzadniya, A. Assessment of Manganese-Zinc Ferrite Nanoparticles as a Novel Magnetic Resonance Imaging Contrast Agent for the Detection of 4T1 Breast Cancer Cells. J. Med. Signals Sens. 2019, 9, 245–251. [Google Scholar] [CrossRef] [PubMed]










| x Values | Crystallite Size | Lattice Constant | (emu/g) | Tc (°C) |
|---|---|---|---|---|
| 0.00 | 32 | 8.462 | 55 | 261 |
| 0.02 | 33 | 8.466 | 58 | - |
| 0.04 | 30 | 8.462 | 56 | 238 |
| 0.06 | 28 | 8.466 | - | - |
| 0.08 | 36 | 8.466 | 54 | - |
| 0.10 | 23 | 8.457 | 48 | 183 |
| 0.15 | - | - | 45 | - |
| x Values | |||
|---|---|---|---|
| 0.00 | 1.73 ± 0.16 | 263 ± 31 | 152 |
| 0.02 | 2.05 ± 0.24 | 300 ± 70 | 146 |
| 0.08 | 5.9 ± 0.71 | 207 ± 19 | 35 |
| 0.10 | 8.4 ± 1.2 | 157 ± 16 | 18.7 |
| 0.15 | 11.6 ± 1.44 | 130 ± 16 | 11.2 |
| Reference | Structure | Ms (emu/g) | ||
|---|---|---|---|---|
| Zahraee et al. [65] | MZF@Citric acid | 53 | 4 | 132 |
| Zahraee et al. [65] | MZF-PEG | 49 | 3 | 79 |
| Junzhao et al. [66] | MZF@4sPCL-b-P(MEO2MA-co-OEGMA) | 59 | 1.28 | 138 |
| Tayebe et al. [67] | MZF@PEG | - | - | 88 |
| Soraya et al. [30] | MZF@PEG | 60 | - | 314 |
| Name | Structure | Dh(nm) | B0(T) | r2/r1 | ||
|---|---|---|---|---|---|---|
| Resovist | Carboxydextran-coated USPIOs | 60 | 3 | 4.6 | 143 | 31.08 |
| Feridex | Dextran-coated SPIOs | 150 | 3 | 4.1 | 93 | 22.68 |
| Gadomer | Gd-DTOA | 30 | 3 | 13 | 23 | 1.76 |
| Magnevist | Gd-DTPA | - | 3 | 3.1 | 3.7 | 1.19 |
| Ferucarbotran® (SHU-555A) | Carboxydextran-coated USPIOs | 60 | 3 | 7.3 | 57 | 7.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeidi, H.; Mozaffari, M.; Ilbey, S.; Dutz, S.; Zahn, D.; Azimi, G.; Bock, M. Effect of Europium Substitution on the Structural, Magnetic and Relaxivity Properties of Mn-Zn Ferrite Nanoparticles: A Dual-Mode MRI Contrast-Agent Candidate. Nanomaterials 2023, 13, 331. https://doi.org/10.3390/nano13020331
Saeidi H, Mozaffari M, Ilbey S, Dutz S, Zahn D, Azimi G, Bock M. Effect of Europium Substitution on the Structural, Magnetic and Relaxivity Properties of Mn-Zn Ferrite Nanoparticles: A Dual-Mode MRI Contrast-Agent Candidate. Nanomaterials. 2023; 13(2):331. https://doi.org/10.3390/nano13020331
Chicago/Turabian StyleSaeidi, Hamidreza, Morteza Mozaffari, Serhat Ilbey, Silvio Dutz, Diana Zahn, Gholamhassan Azimi, and Michael Bock. 2023. "Effect of Europium Substitution on the Structural, Magnetic and Relaxivity Properties of Mn-Zn Ferrite Nanoparticles: A Dual-Mode MRI Contrast-Agent Candidate" Nanomaterials 13, no. 2: 331. https://doi.org/10.3390/nano13020331