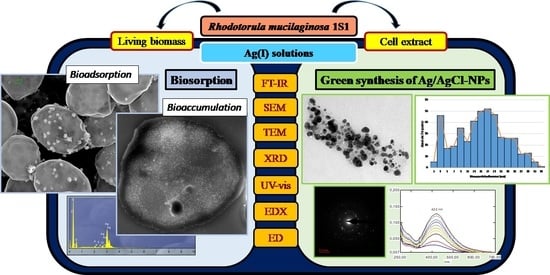
Ag(I) Biosorption and Green Synthesis of Silver/Silver Chloride Nanoparticles by Rhodotorula mucilaginosa 1S1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Biomass, the Cell Extract and Biosorption Tests
2.2. Experimental Design
2.3. Silver Biosorption Kinetics
2.4. Biosorption Equilibrium Study
2.5. Temperature and Thermodynamics Study
2.6. Determination of Biosorption Mechanisms
2.7. Synthesis of Silver Nanoparticles
3. Results and Discussion
3.1. Experimental Design: Optimization of the Factors Involved
3.2. Biosorption Kinetics
3.3. Biosorption Isotherms
3.4. Thermodynamics of the Biosorption Process
3.5. Study of Biosorption Mechanisms
3.6. Synthesis of Silver Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priyadarshanee, M.; Das, S. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104686. [Google Scholar] [CrossRef]
- Rebello, S.; Sivaprasad, M.S.; Anoopkumar, A.N.; Jayakrishnan, L.; Aneesh, E.M.; Narisetty, V.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Pandey, A. Cleaner technologies to combat heavy metal toxicity. J. Environ. Manag. 2021, 296, 113231. [Google Scholar] [CrossRef] [PubMed]
- Tappin, A.D.; Barriada, J.L.; Braungardt, C.B.; Evans, E.H.; Patey, M.D.; Achterberg, E.P. Dissolved silver in European estuarine and coastal waters. Water Res. 2010, 44, 4204–4216. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, R.B.; Patil, S.V.; Patil, C.D.; Salunke, B.K. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol. Res. 2011, 109, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, X.; Wang, B.; Mohamad, O.; Wei, G. Bioaccumulation characterization of zinc and cadmium by Streptomyces zinciresistens, a novel actinomycete. Ecotoxicol. Environ. Saf. 2012, 77, 7–17. [Google Scholar] [CrossRef]
- Yoo-iam, M.; Chaichana, R.; Satapanajaru, T. Toxicity bioaccumulation and biomagnification of silver nanoparticles in green algae (Chlorella sp.), water flea (Moina macrocopa), blood worm (Chironomus spp.) and silver barb (Barbonymus gonionotus). Chem. Speciat. Bioavailab. 2014, 26, 257–265. [Google Scholar] [CrossRef]
- Kennedy, C.J. The Toxicology of Metals in Fishes. In Encyclopedia of Fish Physiology: From Genome to Environment; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; Volume 3, pp. 2061–2068. [Google Scholar]
- Akter, M.; Sikder, T.; Rahman, M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Khan, M.S.; Maqsud, M.S.; Akmal, H.; Umar, A. Chapter 25—Toxicity of silver nanoparticles in the aquatic system. In Nanobiotechnology for Plant Protection, Green Synthesis of Silver Nanomaterials; Kamel, A., Abd-Elsalam, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 627–647. ISBN 9780128245088. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 8, 8–16. [Google Scholar] [CrossRef]
- Das, T.K.; Remanan, S.; Ghosh, S.; Das, N.C. An environment friendly free-standing cellulose membrane derived for catalytic reduction of 4-nitrophenol: A sustainable approach. J. Environ. Chem. Eng. 2021, 9, 104596. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Ruiz, E.; Abriouel, H.; Gálvez, A.; Ezzouhri, L.; Lairini, K.; Espínola, F. Heavy metal tolerance of microorganisms isolated from wastewaters: Identification and evaluation of its potential for biosorption. Chem. Eng. J. 2012, 210, 325–332. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E. Biosorption of Ag(I) from aqueous solutions by Klebsiella sp. 3S1. J. Hazard. Mater. 2017, 329, 166–177. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistecies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Yu, L. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Olawale, S.A.; Okafor, C.C. Comparing the Different Methods Used in the Determination of Thermodynamic Parameters Using Adsorption of Pb(II) on to Chicken Feather as an Example. J. Mater. Sci. Res. Rev. 2020, 6, 1–11. Available online: https://journaljmsrr.com/index.php/JMSRR/article/view/30149 (accessed on 25 November 2021).
- Arshadi, M.; Amiri, M.J.; Mousavi, S. Kinetic, equilibrium and thermodynamic investigations of Ni(II), Cd(II), Cu(II) and Co(II) adsorption on barley straw ash. Water Resour. Ind. 2014, 6, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Zhou, K.; Liu, X.; Liu, F.; Chen, Q. Development of Mg–Al–La tri-metal mixed oxide entrapped in alginate for removal of fluoride from wastewater. RSC Adv. 2017, 7, 31221. [Google Scholar] [CrossRef] [Green Version]
- Harrachea, Z.; Abbasa, M.; Aksila, T.; Trarib, M. Thermodynamic and kinetics studies on adsorption of Indigo Carmine from aqueous solution by activated carbon. Microchem. J. 2019, 144, 180–189. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. An insight into thermodynamics of adsorptive removal of fluoride by calcined Ca–Al–(NO3) layered double hydroxide. RSC Adv. 2015, 5, 105889. [Google Scholar] [CrossRef]
- Barakan, S.; Aghazadeh, V.; Beyragh, A.S.; Mohammadi, S. Thermodynamic, kinetic and equilibrium isotherm studies of As(V) adsorption by Fe(III)-impregnated bentonite. Environ. Dev. Sustain. 2020, 22, 5273–5295. [Google Scholar] [CrossRef]
- Xue, F.; Xu, Y.; Lu, S.; Ju, S.; Xing, W. Adsorption of Cefocelis Hydrochloride on Macroporous Resin: Kinetics, Equilibrium, and Thermodynamic Studies. J. Chem. Eng. Data 2016, 61, 2179–2185. [Google Scholar] [CrossRef]
- Raghav, S.; Kumar, D. Adsorption Equilibrium, Kinetics, and Thermodynamic Studies of Fluoride Adsorbed by Tetrametallic Oxide Adsorbent. J. Chem. Eng. Data 2018, 63, 1682–1697. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Castro, E. Biosorption mechanisms of Ag(I) and the synthesis of nanoparticles by the biomass from Botryosphaeria rhodina MAMB-05. J. Hazard. Mater. 2021, 420, 126598. [Google Scholar] [CrossRef] [PubMed]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala. Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Cleaner Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X. Kinetics and Thermodynamics of Efficient Phosphorus Removal by a Composite Fiber. Appl. Sci. 2019, 9, 2220. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, H.; Chen, X.; Yang, M.; Qi, Y. Selective adsorption of silver ions from aqueous solution using polystyrene-supported trimercaptotriazine resin. J. Environ. Sci. 2012, 24, 2166–2172. [Google Scholar] [CrossRef]
- Liu, X.; Yang, L.; Luo, X.; Pei, J.; Xi, Y.; Liu, C.; Liu, L. A novel non-imprinted adsorbent with superior selectivity towards high performance capture of Ag(I). Chem. Eng. J. 2018, 348, 224–231. [Google Scholar] [CrossRef]
- Kadukova, J. Surface sorption and nanoparticle production as a silver detoxification mechanism of the freshwater alga Parachlorella kessleri. Bioresour. Technol. 2016, 216, 406–413. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, M.T.; El-Sayed, A.S.A. Biocidal Activity of Metal Nanoparticles Synthesized by Fusarium solani against Multidrug-Resistant Bacteria and Mycotoxigenic Fungi. J. Microbiol. Biotechnol. 2020, 30, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.M.; Dumas, P. From structure to cellular mechanism with infrared microspectroscopy. Curr. Opin. Struct. Biol. 2010, 20, 649–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eugenio, M.; Muller, N.; Frasés, S.; Almeida-Paes, R.; Lima, L.M.T.R.; Lemgruber, L.; Farina, M.; De Souza, W.; Sant´Anna, C. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Adv. 2016, 6, 9893–9904. [Google Scholar] [CrossRef] [Green Version]
- Spagnoletti, F.N.; Spedalieri, C.; Kronberg, F.; Giacometti, R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manag. 2019, 231, 457–466. [Google Scholar] [CrossRef]
- Kubasheva, Z.; Sprynskyy, M.; Railean-Plugaru, V.; Pomastowski, P.; Ospanova, A.; Buszewski, B. Synthesis and Antibacterial Activity of (AgCl,Ag)NPs/Diatomite Hybrid Composite. Materials 2020, 13, 3409. [Google Scholar] [CrossRef]
- Ramírez-Rosas, S.L.; Delgado-Alvarado, E.; Sanchez-Vargas, L.O.; Herrera-May, A.L.; Peña-Juarez, M.G.; Gonzalez-Calderon, J.A. Green Route to Produce Silver Nanoparticles Using the Bioactive Flavonoid Quercetin as a Reducing Agent and Food Anti-Caking Agents as Stabilizers. Nanomaterials 2022, 12, 3545. [Google Scholar] [CrossRef]
- Barabadi, H.; Honary, S.; Ebrahimi, P.; Alizadeh, A.; Naghibi, F.; Saravanan, M. Optimization of myco-synthesized silver nanoparticles by response Surface methodology employing Box-Behnken design. Inorg. Nano Met. Chem. 2019, 49, 33–43. [Google Scholar] [CrossRef]








| Lagergren’s Model | Ho’s Model | |
|---|---|---|
| a (mg/g) | −58.73 | −72.03 |
| b (K−1) | 0.379 | 0.426 |
| k0 (g/(mg min)) | 95.244 | 0.2028 |
| Ea (J/mol) | 15,229.73 | 8725.96 |
| R2 | 0.900 | 0.957 |
| ε (mg/g) | 2.63 | 1.93 |
| CV (%) | 5.05 | 3.69 |
| R2 = coefficient of determination ε = standard deviation of the model CV = coefficient of variation (relative error) | ||
| Langmuir | Freundlich | Temkin | Elovich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p-value | p-value | p-value | p-value | ||||||||
| a | −1.120 | 0.0605 | a | 0.7379 | <0.0001 | a | −0.1116 | 0.2214 | a | −2.058 | 0.0497 |
| d | 435.1 | 0.0178 | d | −210.5 | <0.0001 | d | 50.56 | 0.0704 | d | 700.8 | 0.0319 |
| p | 7.25 × 10−4 | 0.0472 | p | 0.1112 | <0.0001 | p | 0.0145 | 0.0705 | p | 4.68 × 10−4 | 0.1953 |
| r | −0.1979 | 0.0669 | r | −30.62 | <0.0001 | r | −3.987 | 0.0905 | r | −0.1233 | 0.2586 |
| ε | 9.5 | ε | 6.08 | ε | 6.18 | ε | 29.19 | ||||
| CV(%) | 14.11 | CV(%) | 8.05 | CV(%) | 9.77 | CV(%) | 16.08 | ||||
| R2 | 0.776 | R2 | 0.934 | R2 | 0.881 | R2 | 0.917 | ||||
| T (°C) | Kd′ (L/g) | ΔH (kJ/mol) | ΔS, (kJ/(mol K)) | ΔG (kJ/mol) |
|---|---|---|---|---|
| 19 | 2.343 | 126.00 | 0.4381 | −1.989 |
| 27 | 8.502 | - | - | −5.493 |
| 37 | 47.316 | - | - | −9.874 |
| R2 = 0.999 | - | - | - | |
| - | - | - | - | - |
| T (°C) | Kd | ΔH (kJ/mol) | ΔS, (kJ/(mol K)) | ΔG (kJ/mol) |
| 19 | 0.7030 | 126.00 | 0.4281 | 0.9359 |
| 27 | 2.551 | - | - | −2.489 |
| 37 | 14.195 | - | - | −6.769 |
| R2 = 0.999 | - | - | - | |
| - | - | - | ||
| T (°C) | Kc | ΔH (kJ/mol) | ΔS, (kJ/(mol K)) | ΔG (kJ/mol) |
| 19 | 123.56 | 133.23 | 0.4976 | −12.136 |
| 27 | 888.54 | - | - | −16.117 |
| 37 | 3061.92 | - | - | −21.093 |
| R2 = 0.968 | - | - | - | |
| - | - | - | - | - |
| n | T (°C) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS, (kJ/(mol K)) |
| 1.859 | 19 | −4.517 | 83.87 | 0.3024 |
| 2.749 | 27 | −6.860 | - | - |
| 3.860 | 37 | −9.955 | - | - |
| - | R2 = 1.0 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, A.J.; Espínola, F.; Ruiz, E.; Moya, M.; Castro, E. Ag(I) Biosorption and Green Synthesis of Silver/Silver Chloride Nanoparticles by Rhodotorula mucilaginosa 1S1. Nanomaterials 2023, 13, 295. https://doi.org/10.3390/nano13020295
Muñoz AJ, Espínola F, Ruiz E, Moya M, Castro E. Ag(I) Biosorption and Green Synthesis of Silver/Silver Chloride Nanoparticles by Rhodotorula mucilaginosa 1S1. Nanomaterials. 2023; 13(2):295. https://doi.org/10.3390/nano13020295
Chicago/Turabian StyleMuñoz, Antonio J., Francisco Espínola, Encarnación Ruiz, Manuel Moya, and Eulogio Castro. 2023. "Ag(I) Biosorption and Green Synthesis of Silver/Silver Chloride Nanoparticles by Rhodotorula mucilaginosa 1S1" Nanomaterials 13, no. 2: 295. https://doi.org/10.3390/nano13020295