Electrochemical Synthesis of Nb-Doped BaTiO3 Nanoparticles with Titanium-Niobium Alloy as Electrode
Abstract
:1. Introduction
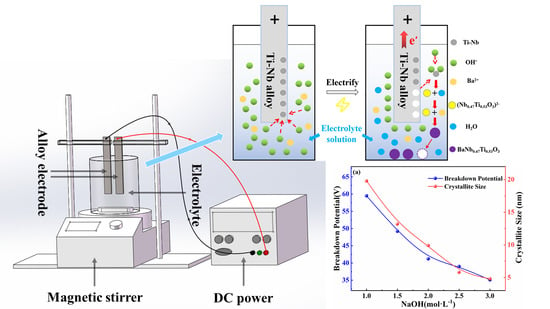
2. Materials and Methods
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tao, J.; Ma, J.; Wang, Y.; Zhu, X.; Liu, J.; Jiang, X.; Lin, B.; Ren, Y. Synthesis of barium titanate nanoparticles via a novel electrochemical route. Mater. Res. Bull. 2008, 43, 639–644. [Google Scholar] [CrossRef]
- Xia, B.; Lenggoro, I.W.; Okuyama, K. Novel Route to Nanoparticle Synthesis by Salt-Assisted Aerosol Decomposition. Adv. Mater. 2001, 13, 1579–1582. [Google Scholar] [CrossRef]
- Boccardi, F.; Heath, R.W., Jr.; Lozano, A.; Marzetta, T.L.; Popovski, P. Five disruptive technology directions for 5G. IEEE Commun. Mag. 2014, 52, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Wong, P.C.; Luo, S. Flip the Chip. Science 2000, 290, 2269–2270. [Google Scholar] [CrossRef] [PubMed]
- Buscaglia, M.T.; Bassoli, M.; Buscaglia, V.; Alessio, R. Solid-State Synthesis of Ultrafine BaTiO3 Powders from Nanocrystalline BaCO3 and TiO2. J. Am. Ceram. Soc. 2005, 88, 2374–2379. [Google Scholar] [CrossRef]
- Vijatovic, M.M.; Petrovic, J.D.; Bobic, T. Electrical properties of lanthanum doped barium titanate ceramics. Mater. Charact. 2011, 62, 1000–1006. [Google Scholar]
- Durán, P.; Capel, F.; Tartaj, J.; Gutierrez, D.; Moure, C. Heating-rate effect on the BaTiO3 formation by thermal decomposition of metal citrate polymeric precursors. Solid State Ion. 2001, 141, 529–539. [Google Scholar] [CrossRef]
- Ischenko, V.; Pippel, E.; Köferstein, R.; Abicht, H.P.; Woltersdorf, J. Barium titanate via thermal decomposition of Ba,Ti-precursor complexes: The nature of the intermediate phases. Solid State Sci. 2007, 9, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.F.; Shen, Z.G.; Liu, F.T. Preparation and properties of barium titanate nanopowder by conventional and high-gravity reactive precipitation methods. Scr. Mater. 2003, 49, 509–514. [Google Scholar] [CrossRef]
- Zanfir, A.V.; Voicu, G.; Jinga, S.I.; Vasile, E.; Ionita, V. Low-temperature synthesis of BaTiO3 nanopowders. Ceram. Int. 2016, 42, 1672–1678. [Google Scholar] [CrossRef]
- Wang, W.; Cao, L.; Liu, W.; Su, G.; Zhang, W. Low-temperature synthesis of BaTiO3 powders by the sol-gel-hydrothermal method. Ceram. Int. 2013, 39, 7127–7134. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, K.; Wu, Q.; Liu, J.; Qiu, J. Preparation and characterization of monodispersed BaTiO3 nanocrystals by sol–hydrothemal method. J. Cryst. Growth 2013, 363, 300–307. [Google Scholar] [CrossRef]
- Kinoshita, K.; Yamaji, A. Grain-size effects on dielectric properties in barium titanate ceramics. J. Appl. Phys. 1976, 47, 371–373. [Google Scholar] [CrossRef]
- Ding, S.; Song, T.; Yang, X.; Luo, G. Effect of Grain Size of BaTiO3 Ceramics on Dielectric Properties. Ferroelectrics 2010, 402, 55–59. [Google Scholar] [CrossRef]
- Hoshina, T.; Takizawa, K.; Li, J.; Kasama, T.; Kakemoto, H.; Tsurumi, T. Domain Size Effect on Dielectric Properties of Barium Titanate Ceramics. Jap. J. Appl. Phys. 2008, 47, 7607–7611. [Google Scholar] [CrossRef]
- Zhao, Z.; Buscaglia, V.; Viviani, M.; Buscaglia, M.T.; Mitoseriu, L.; Testino, A.; Nygren, M.; Johnsson, M.; Nanni, P. Grain-size effects on the ferroelectric behavior of dense nanocrystalline BaTiO3 ceramics. Phys. Rev. B 2004, 70, 024107. [Google Scholar] [CrossRef]
- Santos, G.O.S.; Silva, R.S.; Costa, L.P.; Cellet, T.S.P.; Rubira, A.F.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Influence of synthesis conditions on the properties of electrochemically synthesized BaTiO3 nanoparticles. Ceram. Int. 2014, 40, 3603–3609. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Borland, W.J.; Maria, J.P. Enhanced dielectric tunability in barium titanate thin films with boron additions. Scr. Mater. 2008, 58, 549–552. [Google Scholar] [CrossRef]
- Bernard, J.; Houivet, D.; Fallah, J. MgTiO3 for Cu base metal multilayer ceramic capacitors. J. Eur. Ceram. Soc. 2004, 24, 1877–1881. [Google Scholar] [CrossRef]
- Frey, M.H.; Xu, Z.; Han, P.; Payne, D.A. The role of interfaces on an apparent grain size effect on the dielectric properties for ferroelectric barium titanate ceramics. Ferroelectrics 1998, 206, 337–353. [Google Scholar] [CrossRef]
- Hennings, D.F.K.; Schreinemacher, H. Ca-acceptors in dielectric ceramics sintered in reducive atmospheres. J. Eur. Ceram. Soc. 1995, 15, 795–800. [Google Scholar] [CrossRef]
- Panwar, N.S.; Semwal, B.S. Dielectric properties of BaTiO3 and La doped BaTiO3 ceramics. Pramana 1991, 36, 163–166. [Google Scholar] [CrossRef]
- Ren, P.; He, J.; Wang, X. Colossal permittivity in niobium doped BaTiO3 ceramics annealed in N2. Scr. Mater. 2018, 146, 110–114. [Google Scholar] [CrossRef]
- Paunovic, V.; Mitic, V.V.; Djordjevic, M.; Prijic, Z. Niobium doping effect on BaTiO3 structure and dielectric properties. Ceram. Int. 2020, 46, 8154–8164. [Google Scholar] [CrossRef]
- Dechakupt, T.; Tangsritrakul, J.; Ketsuwan, P.; Yimnirun, R. Microstructure and Electrical Properties of Niobium Doped Barium Titanate Ceramics. Ferroelectrics 2011, 415, 141–148. [Google Scholar] [CrossRef]
- Urek, S.; Drofenik, M. PTCR behaviour of highly donor doped BaTiO3. J. Eur. Ceram. Soc. 1999, 19, 913–916. [Google Scholar] [CrossRef]
- Chen, A.; Zhi, Y.; Zhi, J. Synthesis and characterization of Ba(Ti1−xCex)O3 ceramics. J. Eur. Ceram. Soc. 1997, 17, 1217–1221. [Google Scholar] [CrossRef]
- Hofer, C.; Meyer, R.; Böttger, U.; Waser, R. Characterization of Ba(Ti, Zr)O3 ceramics sintered under reducing conditions. J. Eur. Ceram. Soc. 2004, 24, 1473–1477. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wu, S.Y.; Xu, P. Studies on the local structures and spin Hamiltonian parameters for the rhombic Nb4+ centers in MO2(M= Sn, Ti and Ge) crystals. Eur. Phys. J. Appls. 2011, 53, 20902. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, D.; Feng, G. Effects of Ni2+, Zr4+, or Ti4+ doping on the electromagnetic and microwave absorption properties of CaMnO3 particles. Ceram. Int. 2021, 47, 19995–20002. [Google Scholar] [CrossRef]
- Chen, W.; Liang, H.; Shao, L.; Shu, J.; Wang, Z. Observation of the structural changes of sol-gel formed Li2MnTi3O8 during electrochemical reaction by in-situ and ex-situ studies. Electrochim. Acta 2015, 152, 187–194. [Google Scholar] [CrossRef]
- Yan, L.; Chen, G.; Sarker, S.; Richins, S.; Wang, H.; Xu, W.; Rui, X.; Luo, H. Ultrafine Nb2O5 Nanocrystal Coating on Reduced Graphene Oxide as Anode Material for High Performance Sodium Ion Battery. ACS Appl. Mater. Inter. 2016, 8, 22213–22219. [Google Scholar] [CrossRef]
- Zeng, J.L.; Teng, H.P.; Lu, F.H. Electrochemical deposition of barium titanate thin films on TiN/Si substrates. Surf. Coat. Tech. 2013, 231, 297–300. [Google Scholar] [CrossRef]
- Macova, Z.; Bouzek, K.; Híveš, J. Research progress in the electrochemical synthesis of ferrate (VI). Electrochim. Acta 2009, 54, 2673–2683. [Google Scholar] [CrossRef]
- Schaafsma, S.H.; Vonk, P.; Segers, P.; Kossen, N.W. Description of agglomerate growth. Powder Technol. 1998, 97, 183–190. [Google Scholar] [CrossRef]









| Breakdown Potential (V) | NaOH (mol·L−1) | Initial Current (I) | Crystallite Size (nm) |
|---|---|---|---|
| 59.5 | 1 | 3.25 | 19.8 |
| 49.2 | 1.5 | 3.25 | 13.2 |
| 41.2 | 2 | 3.25 | 9.9 |
| 39.0 | 2.5 | 3.25 | 5.8 |
| 35.1 | 3 | 3.25 | 4.8 |
| NaOH (mol·L−1) | a (Å) | V = a3 (Å3) | Crystallite Size (nm) |
|---|---|---|---|
| 1 | 4.043 ± 0.010 | 66.1 ± 0.5 | 20.3 ± 1.6 |
| 2 | 4.059 ± 0.005 | 66.9 ± 0.2 | 10.5 ± 1.5 |
| 3 | 4.063 ± 0.006 | 67.1 ± 0.3 | 5.4 ± 1.0 |
| Elements | Weight (%) | Atomic (%) |
|---|---|---|
| O K | 30.11 | 71.04 |
| Na K | 1.96 | 3.05 |
| Ti K | 12.59 | 9.37 |
| Nb L | 17.6 | 6.75 |
| Ba L | 37.74 | 9.79 |
| Totals | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Q.; Hu, W.; Wang, T.; Wang, S.; Liu, G.; Han, X.; Guo, F.; Fan, Y. Electrochemical Synthesis of Nb-Doped BaTiO3 Nanoparticles with Titanium-Niobium Alloy as Electrode. Nanomaterials 2023, 13, 252. https://doi.org/10.3390/nano13020252
Yuan Q, Hu W, Wang T, Wang S, Liu G, Han X, Guo F, Fan Y. Electrochemical Synthesis of Nb-Doped BaTiO3 Nanoparticles with Titanium-Niobium Alloy as Electrode. Nanomaterials. 2023; 13(2):252. https://doi.org/10.3390/nano13020252
Chicago/Turabian StyleYuan, Qi, Wencai Hu, Tao Wang, Sen Wang, Gaobin Liu, Xueyan Han, Feixiang Guo, and Yongheng Fan. 2023. "Electrochemical Synthesis of Nb-Doped BaTiO3 Nanoparticles with Titanium-Niobium Alloy as Electrode" Nanomaterials 13, no. 2: 252. https://doi.org/10.3390/nano13020252