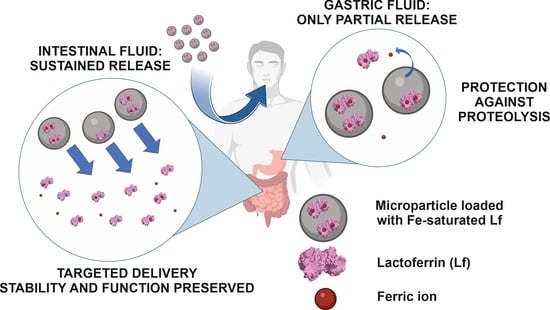
Encapsulation of Iron-Saturated Lactoferrin for Proteolysis Protection with Preserving Iron Coordination and Sustained Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Iron Saturation Determination
2.2. Protein Encapsulation
2.3. Microparticles Characterization
2.4. Protein Release from the MPs
2.5. Protein Stability and Functionality Studies
3. Results and Discussion
3.1. Microparticles Synthesis and Characterization
3.2. Protein Release from the MPs
3.3. Characterization of Lf Released from MPs
3.3.1. Iron Saturation Level
3.3.2. Structural Stability and Iron Scavenging Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno-Expósito, L.; Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Ramos-Torrecillas, J.; de Luna-Bertos, E. Multifunctional capacity and therapeutic potential of lactoferrin. Life Sci. 2018, 195, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; Delagarza, M. Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Cutone, A.; Lepanto, M.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.F.; Zubair, D.; Bashir, M.N.; Alagawany, M.; Ahmed, S.; Shah, Q.A.; Buzdar, J.A.; Arain, M.A. Nutraceutical and Health-Promoting Potential of Lactoferrin, an Iron-Binding Protein in Human and Animal: Current Knowledge. Biol. Trace Elem. Res. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 Å resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, L.; Mu, Z.; Liu, L.; Yang, J.; Wu, Z.; Pan, D.; Liu, L. Research Advances of Lactoferrin in Electrostatic Spinning, Nano Self-Assembly, and Immune and Gut Microbiota Regulation. J. Agric. Food Chem. 2022, 70, 10075–10089. [Google Scholar] [CrossRef]
- Aisen, P.; Leibman, A. Lactoferrin and transferrin: A comparative study. BBA—Protein Struct. 1972, 257, 314–323. [Google Scholar] [CrossRef]
- Gruden, Š.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Gajda-Morszewski, P.; Brindell, M. Lactoferrin as a Potent Natural Supplement Exhibiting a Synergistic Effect with Drugs in Antimicrobial and Anticancer Therapies. Curr. Protein Pept. Sci. 2021, 22, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Diarra, M.S.; Petitclerc, D.; Lacasse, P. Effect of lactoferrin in combination with penicillin on the morphology and the physiology of Staphylococcus aureus isolated from bovine mastitis. J. Dairy Sci. 2002, 85, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.J.; Oguejiofor, W.; Price, R.; Shur, J. Investigation of the enhanced antimicrobial activity of combination dry powder inhaler formulations of lactoferrin. Int. J. Pharm. 2016, 514, 399–406. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Palmano, K.P.; Sun, X.; Kanwar, R.K.; Gupta, R.; Haggarty, N.; Rowan, A.; Ram, S.; Krissansen, G.W. “Iron-saturated” lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy. Immunol. Cell Biol. 2008, 86, 277–288. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, R.; Przepiorski, A.; Reddy, S.; Palmano, K.P.; Krissansen, G.W. “Iron-saturated” bovine lactoferrin improves the chemotherapeutic effects of tamoxifen in the treatment of basal-like breast cancer in mice. BMC Cancer 2012, 12, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Bellés, A.; Aguirre-Ramírez, D.; Abad, I.; Parras-Moltó, M.; Sánchez, L.; Grasa, L. Lactoferrin modulates gut microbiota and Toll-like receptors (TLRs) in mice with dysbiosis induced by antibiotics. Food Funct. 2022, 13, 5854–5869. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Duncan, S.E.; Lesser, G.J.; Ray, W.K.; Dietrich, A.M. Effect of lactoferrin on taste and smell abnormalities induced by chemotherapy: A proteome analysis. Food Funct. 2018, 9, 4948–4958. [Google Scholar] [CrossRef] [PubMed]
- Moastafa, T.M.; El-Sissy, A.E.-D.E.; El-Saeed, G.K.; Koura, M.S.E.-D. Study on the Therapeutic Benefit on Lactoferrin in Patients with Colorectal Cancer Receiving Chemotherapy. Int. Sch. Res. Notices 2014, 2014, 184278. [Google Scholar] [CrossRef]
- European Commission. European Commission Implementing Decision No. 2012/727/EU Authorizing the Placing on the Market of Bovine Lactoferrin as a Novel Food Ingredient under Regulation (EC) No. 258/97 of the European Parliament and of the Council; European Commission: Strasbourg, France, 2012. [Google Scholar]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef]
- Yao, X.; Bunt, C.; Cornish, J.; Quek, S.-Y.; Wen, J. Stability of Bovine Lactoferrin in Luminal Extracts and Mucosal Homogenates from Rat Intestine: A Prelude to Oral Absorption. Chem. Biol. Drug Des. 2014, 84, 676–684. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Mild thermal treatment and in-vitro digestion of three forms of bovine lactoferrin: Effects on functional properties. Int. Dairy J. 2017, 64, 22–30. [Google Scholar] [CrossRef]
- Shimoni, G.; Shani Levi, C.; Levi Tal, S.; Lesmes, U. Emulsions stabilization by lactoferrin nano-particles under in vitro digestion conditions. Food Hydrocoll. 2013, 33, 264–272. [Google Scholar] [CrossRef]
- David-Birman, T.; Mackie, A.; Lesmes, U. Impact of dietary fibers on the properties and proteolytic digestibility of lactoferrin nano-particles. Food Hydrocoll. 2013, 31, 33–41. [Google Scholar] [CrossRef]
- Grosvenor, A.J.; Haigh, B.J.; Dyer, J.M. Digestion proteomics: Tracking lactoferrin truncation and peptide release during simulated gastric digestion. Food Funct. 2014, 5, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Troost, F.J.; Saris, W.H.M.; Brummer, R.-J.M. Orally Ingested Human Lactoferrin Is Digested and Secreted in the Upper Gastrointestinal Tract In Vivo in Women with Ileostomies. J. Nutr. 2002, 132, 2597–2600. [Google Scholar] [CrossRef]
- Kuwata, H.; Yamauchi, K.; Teraguchi, S.; Ushida, Y.; Shimokawa, Y.; Toida, T.; Hayasawa, H. Functional Fragments of Ingested Lactoferrin Are Resistant to Proteolytic Degradation in the Gastrointestinal Tract of Adult Rats. J. Nutr. 2001, 131, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Furlund, C.B.; Ulleberg, E.K.; Devold, T.G.; Flengsrud, R.; Jacobsen, M.; Sekse, C.; Holm, H.; Vegarud, G.E. Identification of lactoferrin peptides generated by digestion with human gastrointestinal enzymes. J. Dairy Sci. 2013, 96, 75–88. [Google Scholar] [CrossRef]
- Fan, F.; Shi, P.; Chen, H.; Tu, M.; Wang, Z.; Lu, W.; Du, M. Identification and availability of peptides from lactoferrin in the gastrointestinal tract of mice. Food Funct. 2019, 10, 879–885. [Google Scholar] [CrossRef]
- Troost, F.J.; Steijns, J.; Saris, W.H.M.; Brummer, R.-J.M. Gastric Digestion of Bovine Lactoferrin In Vivo in Adults. J. Nutr. 2001, 131, 2101–2104. [Google Scholar] [CrossRef]
- Gleeson, J.P.; Fein, K.C.; Chaudhary, N.; Doerfler, R.; Newby, A.N.; Whitehead, K.A. The enhanced intestinal permeability of infant mice enables oral protein and macromolecular absorption without delivery technology. Int. J. Pharm. 2021, 593, 120120. [Google Scholar] [CrossRef]
- Dupont, T.L. Donor Milk Compared with Mother’s Own Milk. In Hematology, Immunology and Genetics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–52. [Google Scholar]
- Abdallah, F.B.; el Hage Chahine, J.-M. Transferrins: Iron release from lactoferrin. J. Mol. Biol. 2000, 303, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ong, R.; Cornish, J.; Wen, J. Nanoparticular and other carriers to deliver lactoferrin for antimicrobial, antibiofilm and bone-regenerating effects: A review. BioMetals 2022, 36, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Abad, I.; Conesa, C.; Sánchez, L. Development of Encapsulation Strategies and Composite Edible Films to Maintain Lactoferrin Bioactivity: A Review. Materials 2021, 14, 7358. [Google Scholar] [CrossRef]
- Guan, R.; Ma, J.; Wu, Y.; Lu, F.; Xiao, C.; Jiang, H.; Kang, T. Development and characterization of lactoferrin nanoliposome: Cellular uptake and stability. Nanoscale Res. Lett. 2012, 7, 679. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Sakai, H.; Tokunaga, S.; Sharmin, T.; Aida, T.M.; Mishima, K. Encapsulation of Lactoferrin for Sustained Release Using Particles from Gas-Saturated Solutions. Processes 2020, 9, 73. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Javier Otero-Espinar, F. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur. J. Pharm. Biopharm. 2022, 172, 144–156. [Google Scholar] [CrossRef]
- Gracia, R.; Yus, C.; Abian, O.; Mendoza, G.; Irusta, S.; Sebastian, V.; Andreu, V.; Arruebo, M. Enzyme structure and function protection from gastrointestinal degradation using enteric coatings. Int. J. Biol. Macromol. 2018, 119, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. GRAS Notice (GRN) No. 710; Food and Drug Administration: Baltimore, MD, USA, 2017.
- European Commission. European Commission Regulation No. 1129/2011 amending Annex II to Regulation (EC) No. 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Patra, C.h.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Futur. J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Tarcha, P. Polymers for Controlled Drug Delivery, 1st ed.; CRC Press: Boston, MA, USA, 1991. [Google Scholar]
- Eisele, J.; Haynes, G.; Rosamilia, T. Characterisation and toxicological behaviour of Basic Methacrylate Copolymer for GRAS evaluation. Regul. Toxicol. Pharmacol. 2011, 61, 32–43. [Google Scholar] [CrossRef]
- Gajda-Morszewski, P.; Śpiewak-Wojtyła, K.; Oszajca, M.; Brindell, M. Strategies for oral delivery of metal-saturated lactoferrin. Curr. Protein Pept. Sci. 2019, 20, 1046–1051. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Mahidhara, G.; Kanwar, R.K. Novel alginate-enclosed chitosan-calcium phosphate-loaded iron-saturated bovine lactoferrin nanocarriers for oral delivery in colon cancer therapy. Nanomedicine 2012, 7, 1521–1550. [Google Scholar] [CrossRef] [PubMed]
- Majka, G.; Spiewak, K.; Kurpiewska, K.; Heczko, P.; Stochel, G.; Strus, M.; Brindell, M.; Śpiewak, K.; Kurpiewska, K.; Heczko, P.; et al. A high-throughput method for the quantification of iron saturation in lactoferrin preparations. Anal. Bioanal. Chem. 2013, 405, 5191–5200. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. (1997–2015) ImageJ. National Institutes of Health, Bethesda, Maryland, USA. Available online: http://imagej.nih.gov/ij (accessed on 16 July 2023).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.-H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef] [PubMed]
- Wïniewska, M.; Szewczuk-Karpisz, K.; Sternik, D. Adsorption and thermal properties of the bovine serum albumin-silicon dioxide system. J. Therm. Anal. Calorim. 2015, 120, 1355–1364. [Google Scholar] [CrossRef]
- Brock, J.H. Lactoferrin Structure-Function Relationships. Lactoferrin 1997, 28, 3–23. [Google Scholar] [CrossRef]
- Mahidhara, G.; Kanwar, R.K.; Roy, K.; Kanwar, J.R. Oral administration of iron-saturated bovine lactoferrin–loaded ceramic nanocapsules for breast cancer therapy and influence on iron and calcium metabolism. Int. J. Nanomed. 2015, 10, 4081–4098. [Google Scholar] [CrossRef]
- Kiryukhin, M.V.; Lim, S.H.; Lau, H.H.; Antipina, M.; Khin, Y.W.; Chia, C.Y.; Harris, P.; Weeks, M.; Berry, C.; Hurford, D.; et al. Surface-reacted calcium carbonate microparticles as templates for lactoferrin encapsulation. J. Colloid Interface Sci. 2021, 594, 362–371. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Eriksen, E.K.; Holm, H.; Jensen, E.; Aaboe, R.; Devold, T.G.; Jacobsen, M.; Vegarud, G.E. Different digestion of caprine whey proteins by human and porcine gastrointestinal enzymes. Br. J. Nutr. 2010, 104, 374–381. [Google Scholar] [CrossRef]
- Inglingstad, R.A.; Devold, T.G.; Eriksen, E.K.; Holm, H.; Jacobsen, M.; Liland, K.H.; Rukke, E.O.; Vegarud, G.E. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci. Technol. 2010, 90, 549–563. [Google Scholar] [CrossRef]
- Mata, L.; Sánchez, L.; Headon, D.R.; Calvo, M. Thermal Denaturation of Human Lactoferrin and Its Effect on the Ability to Bind Iron. J. Agric. Food Chem. 1998, 46, 3964–3970. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajda-Morszewski, P.; Poznańska, A.; Yus, C.; Arruebo, M.; Brindell, M. Encapsulation of Iron-Saturated Lactoferrin for Proteolysis Protection with Preserving Iron Coordination and Sustained Release. Nanomaterials 2023, 13, 2524. https://doi.org/10.3390/nano13182524
Gajda-Morszewski P, Poznańska A, Yus C, Arruebo M, Brindell M. Encapsulation of Iron-Saturated Lactoferrin for Proteolysis Protection with Preserving Iron Coordination and Sustained Release. Nanomaterials. 2023; 13(18):2524. https://doi.org/10.3390/nano13182524
Chicago/Turabian StyleGajda-Morszewski, Przemysław, Anna Poznańska, Cristina Yus, Manuel Arruebo, and Małgorzata Brindell. 2023. "Encapsulation of Iron-Saturated Lactoferrin for Proteolysis Protection with Preserving Iron Coordination and Sustained Release" Nanomaterials 13, no. 18: 2524. https://doi.org/10.3390/nano13182524