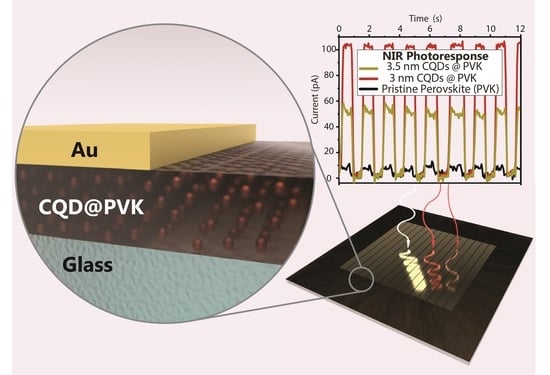
Sub-Bandgap Sensitization of Perovskite Semiconductors via Colloidal Quantum Dots Incorporation
Abstract
:1. Introduction

2. Materials and Methods
2.1. PbS QD Synthesis
2.2. Ligand Exchange
2.3. Film Preparation
2.4. Device Fabrication
2.5. Characterization
3. Results and Discussion
3.1. Structural Properties of the Composite Films
3.2. Optical Assessment of CQD@PVK Films
3.3. Photoconductivity of CQD@PVK Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosokawa, H.; Tamaki, R.; Sawada, T.; Okonogi, A.; Sato, H.; Ogomi, Y.; Hayase, S.; Okada, Y.; Yano, T. Solution-processed intermediate-band solar cells with lead sulfide quantum dots and lead halide perovskites. Nat. Commun. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Ekins-Daukes, N.J.; Kita, T.; Tamaki, R.; Yoshida, M.; Pusch, A.; Hess, O.; Phillips, C.C.; Farrell, D.J.; Ahsan, N.; et al. Intermediate band solar cells: Recent progress and future directions. Appl. Phys. Rev. 2015, 2, 021302. [Google Scholar] [CrossRef]
- Luque, A.; Martí, A. The Intermediate Band Solar Cell: Progress Toward the Realization of an Attractive Concept. Adv. Mater. 2009, 22, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, I.; Martí, A. Intermediate band solar cells: Present and future. Prog. Photovolt. Res. Appl. 2021, 29, 705–713. [Google Scholar] [CrossRef]
- Alexandre, M.; Águas, H.; Fortunato, E.; Martins, R.; Mendes, M.J. Light management with quantum nanostructured dots-in-host semiconductors. Light. Sci. Appl. 2021, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, C.K.; Loi, H.L.; Yan, F. Perovskite-Based Phototransistors and Hybrid Photodetectors. Adv. Funct. Mater. 2020, 30, 1903907. [Google Scholar] [CrossRef]
- Ngo, T.T.; Mora-Seró, I. Interaction between Colloidal Quantum Dots and Halide Perovskites: Looking for Constructive Synergies. J. Phys. Chem. Lett. 2019, 10, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Pattanasattayavong, P.; Anthopoulos, T.D. Metal-Halide Perovskite Transistors for Printed Electronics: Challenges and Opportunities. Adv. Mater. 2017, 29, 1702838. [Google Scholar] [CrossRef]
- Menda, U.D.; Ribeiro, G.; Nunes, D.; Calmeiro, T.; Águas, H.; Fortunato, E.; Martins, R.; Mendes, M.J. High-performance wide bandgap perovskite solar cells fabricated in ambient high-humidity conditions. Mater. Adv. 2021, 2, 6344–6355. [Google Scholar] [CrossRef]
- Brites, M.J.; Barreiros, M.A.; Corregidor, V.; Alves, L.C.; Pinto, J.V.; Mendes, M.J.; Fortunato, E.; Martins, R.; Mascarenhas, J. Ultrafast Low-Temperature Crystallization of Solar Cell Graded Formamidinium-Cesium Mixed-Cation Lead Mixed-Halide Perovskites Using a Reproducible Microwave-Based Process. ACS Appl. Energy Mater. 2019, 2, 1844–1853. [Google Scholar] [CrossRef]
- Ou, Q.; Bao, X.; Zhang, Y.; Shao, H.; Xing, G.; Li, X.; Shao, L.; Bao, Q. Band structure engineering in metal halide perovskite nanostructures for optoelectronic applications. Nano Mater. Sci. 2019, 1, 268–287. [Google Scholar] [CrossRef]
- Cao, F.; Wang, M.; Sun, H.; Tian, W.; Li, L. Ordered array structures for efficient perovskite solar cells. Eng. Rep. 2020, 2, e12319. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Chen, J. Opportunities and challenges of hole transport materials for high-performance inverted hybrid-perovskite solar cells. Exploration 2023, 3, 20220027. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Yang, X.; Zhong, C.; Chen, G.; Yu, C.; Chen, Y.; Wu, T.; Kuo, H.; Lin, Y.; et al. Brightened Bicomponent Perovskite Nanocomposite Based on Förster Resonance Energy Transfer for Micro-LED Displays. Adv. Mater. 2023, 35, e2300834. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Gao, L.; Lu, J.; Ma, C.; Du, Y.; Wang, P.; Ding, Z.; Wang, S.; Xu, P.; Liu, D.; et al. One-stone-for-two-birds strategy to attain beyond 25% perovskite solar cells. Nat. Commun. 2023, 14, 839. [Google Scholar] [CrossRef] [PubMed]
- Best Research-Cell Efficiency Chart|Photovoltaic Research|NREL. Available online: https://www-nrel-gov.translate.goog/pv/cell-efficiency.html?_x_tr_sl=en&_x_tr_tl=pt&_x_tr_hl=pt-PT&_x_tr_pto=sc (accessed on 5 July 2023).
- Song, L. Perovskite solar cells toward industrialization: Screen printed perovskite films. Mater. Rep. Energy 2022, 2, 100171. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiang, H.; Ran, R.; Zhou, W.; Wang, W.; Shao, Z. Beyond two-dimension: One- and zero-dimensional halide perovskites as new-generation passivators for high-performance perovskite solar cells. J. Energy Chem. 2023, 83, 189–208. [Google Scholar] [CrossRef]
- Martí, A.; Antolín, E.; Stanley, C.R.; Farmer, C.D.; López, N.; Díaz, P.; Cánovas, E.; Linares, P.G.; Luque, A. Production of Photocurrent due to Intermediate-to-Conduction-Band Transitions: A Demonstration of a Key Operating Principle of the Intermediate-Band Solar Cell. Phys. Rev. Lett. 2006, 97, 247701. [Google Scholar] [CrossRef]
- Luque, A.; Martí, A.; Nozik, A.J. Solar Cells Based on Quantum Dots: Multiple Exciton Generation and Intermediate Bands. MRS Bull. 2007, 32, 236–241. [Google Scholar] [CrossRef]
- Luque, A.; Martí, A. A metallic intermediate band high efficiency solar cell. Prog. Photovolt. Res. Appl. 2001, 9, 73–86. [Google Scholar] [CrossRef]
- Marsen, B.; Klemz, S.; Unold, T.; Schock, H.-W. Investigation of the Sub-Bandgap Photoresponse in CuGaS2: Fe for Intermediate Band Solar Cells. Prog. Photovolt. Res. Appl. 2012, 20, 625–629. [Google Scholar] [CrossRef]
- Wang, W.; Lin, A.S.; Phillips, J.D.; Metzger, W.K. Generation and recombination rates at ZnTe:O intermediate band states. Appl. Phys. Lett. 2009, 95, 261107. [Google Scholar] [CrossRef]
- Yu, K.M.; Walukiewicz, W.; Wu, J.; Shan, W.; Beeman, J.W.; Scarpulla, M.A.; Dubon, O.D.; Becla, P. Diluted II-VI Oxide Semiconductors with Multiple Band Gaps. Phys. Rev. Lett. 2003, 91, 246403. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.; Clarke, T.M.; MacQueen, R.W.; Cheng, Y.Y.; Trevitt, A.J.; Mozer, A.J.; Wagner, P.; Schmidt, T.W.; Nattestad, A. An intermediate band dye-sensitised solar cell using triplet–triplet annihilation. Phys. Chem. Chem. Phys. 2015, 17, 24826–24830. [Google Scholar] [CrossRef] [PubMed]
- Ekins-Daukes, N.J.; Schmidt, T.W. A molecular approach to the intermediate band solar cell: The symmetric case. Appl. Phys. Lett. 2008, 93, 063507. [Google Scholar] [CrossRef]
- Luque, A.; Martí, A.; Stanley, C.; López, N.; Cuadra, L.; Zhou, D.; Pearson, J.L.; McKee, A. General equivalent circuit for intermediate band devices: Potentials, currents and electroluminescence. J. Appl. Phys. 2004, 96, 903–909. [Google Scholar] [CrossRef]
- Marti, A.; Cuadra, L.; Luque, A. Quantum dot intermediate band solar cell. In Proceedings of the Conference Record of the Twenty-Eighth IEEE Photovoltaic Specialists Conference (Cat. No.00CH37036), Anchorage, AK, USA, 15–22 September 2000; pp. 940–943. [Google Scholar] [CrossRef]
- Mendes, M.J.; Luque, A.; Tobías, I.; Martí, A. Plasmonic light enhancement in the near-field of metallic nanospheroids for application in intermediate band solar cells. Appl. Phys. Lett. 2009, 95, 071105. [Google Scholar] [CrossRef]
- Jasim, K.E. Quantum Dots Solar Cells. In Solar Cells—New Approaches and Reviews; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.R.; Zhao, Y.; Akins, D.L. Synthesis and Characterization of Quantum Dots: A Case Study Using PbS. J. Chem. Educ. 2015, 92, 1860–1865. [Google Scholar] [CrossRef]
- Chi, W.; Banerjee, S.K. Development of perovskite solar cells by incorporating quantum dots. Chem. Eng. J. 2021, 426, 131588. [Google Scholar] [CrossRef]
- Ning, Z.; Gong, X.; Comin, R.; Walters, G.; Fan, F.; Voznyy, O.; Yassitepe, E.; Buin, A.K.; Hoogland, S.; Sargent, E.H. Quantum-dot-in-perovskite solids. Nature 2015, 523, 324–328. [Google Scholar] [CrossRef]
- Moreels, I.; Lambert, K.; Smeets, D.; Muynck, D.D.; Nollet, T.; Martins, J.C.; Vanhaecke, F.; Vantomme, A.; Delerue, C.; Allan, G.; et al. Size-Dependent Optical Properties of Colloidal PbS Quantum Dots. ACS Nano 2009, 3, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.J.; Hernández, E.; López, E.; García-Linares, P.; Ramiro, I.; Artacho, I.; Antolín, E.; Tobías, I.; Martí, A.; Luque, A. Self-organized colloidal quantum dots and metal nanoparticles for plasmon-enhanced intermediate-band solar cells. Nanotechnology 2013, 24, 345402. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.; Martí, A.; Mendes, M.J.; Tobías, I. Light absorption in the near field around surface plasmon polaritons. J. Appl. Phys. 2008, 104, 113118. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, J.; Johansson, E.M.; Zhang, X. PbS Colloidal Quantum Dot Inks for Infrared Solar Cells. iScience 2020, 23, 101753. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.T.; Masi, S.; Mendez, P.F.; Kazes, M.; Oron, D.; Seró, I.M. PbS quantum dots as additives in methylammonium halide perovskite solar cells: The effect of quantum dot capping. Nanoscale Adv. 2019, 1, 4109–4118. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Luo, S.; Yin, X.; Zhou, Y.; Nan, H.; Li, J.; Li, X.; Oron, D.; Shen, H.; Lin, H. Hybrid PbS Quantum-Dot-in-Perovskite for High-Efficiency Perovskite Solar Cell. Small 2018, 14, e1801016. [Google Scholar] [CrossRef]
- Menda, U.D.; Ribeiro, G.; Deuermeier, J.; López, E.; Nunes, D.; Jana, S.; Artacho, I.; Martins, R.; Mora-Seró, I.; Mendes, M.J.; et al. Thermal-Carrier-Escape Mitigation in a Quantum-Dot-In-Perovskite Intermediate Band Solar Cell via Bandgap Engineering. Available online: https://arxiv.org/ftp/arxiv/papers/2302/2302.13305.pdf (accessed on 24 July 2023).
- Caputo, M.; Cefarin, N.; Radivo, A.; Demitri, N.; Gigli, L.; Plaisier, J.R.; Panighel, M.; Di Santo, G.; Moretti, S.; Giglia, A.; et al. Electronic structure of MAPbI3 and MAPbCl3: Importance of band alignment. Sci. Rep. 2019, 9, 15159. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Wan, J.; Tao, H.; Liu, Q.; Xiong, L.; Qin, P.; Wang, J.; Lei, H.; Yang, G.; et al. Efficient hole-blocking layer-free planar halide perovskite thin-film solar cells. Nat. Commun. 2015, 6, 6700. [Google Scholar] [CrossRef]
- Dao, T.D.; Hafez, M.E.; Beloborodov, I.; Jeong, H.-D. Thioacetic-Acid Capped PbS Quantum Dot Solids Exhibiting Thermally Activated Charge Hopping Transport. Bull. Korean Chem. Soc. 2014, 35, 457–465. [Google Scholar] [CrossRef]
- Fan, P.; Gu, D.; Liang, G.-X.; Luo, J.-T.; Chen, J.-L.; Zheng, Z.-H.; Zhang, D.-P. High-performance perovskite CH3NH3PbI3 thin films for solar cells prepared by single-source physical vapour deposition. Sci. Rep. 2016, 6, 29910. [Google Scholar] [CrossRef]
- Wu, C.; Chen, K.; Guo, D.Y.; Wang, S.L.; Li, P.G. Cations substitution tuning phase stability in hybrid perovskite single crystals by strain relaxation. RSC Adv. 2018, 8, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Gaulding, E.A.; Chen, X.; Yang, Y.; Harvey, S.P.; To, B.; Kim, Y.-H.; Beard, M.C.; Sercel, P.C.; Luther, J.M. Embedding PbS Quantum Dots (QDs) in Pb-Halide Perovskite Matrices: QD Surface Chemistry and Antisolvent Effects on QD Dispersion and Confinement Properties. ACS Mater. Lett. 2020, 2, 1464–1472. [Google Scholar] [CrossRef]
- Baranov, D.; Toso, S.; Imran, M.; Manna, L. Investigation into the Photoluminescence Red Shift in Cesium Lead Bromide Nanocrystal Superlattices. J. Phys. Chem. Lett. 2019, 10, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Gerlein, L.F.; Ma, X.; Haughn, C.R.; Doty, M.F.; Cloutier, S.G. Impact of Different Surface Ligands on the Optical Properties of PbS Quantum Dot Solids. Materials 2015, 8, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- Levine, I.; Nayak, P.K.; Wang, J.T.-W.; Sakai, N.; Van Reenen, S.; Brenner, T.M.; Mukhopadhyay, S.; Snaith, H.J.; Hodes, G.; Cahen, D. Interface-Dependent Ion Migration/Accumulation Controls Hysteresis in MAPbI3 Solar Cells. J. Phys. Chem. C 2016, 120, 16399–16411. [Google Scholar] [CrossRef]
- Mićić, O.I.; Ahrenkiel, S.P.; Nozik, A.J. Synthesis of extremely small InP quantum dots and electronic coupling in their disordered solid films. Appl. Phys. Lett. 2001, 78, 4022–4024. [Google Scholar] [CrossRef]
- Teixeira, J.; Salomé, P.; Alves, B.; Edoff, M.; Leitão, J. Evidence of Limiting Effects of Fluctuating Potentials on VOC of Cu(In, Ga)Se2 Thin-Film Solar Cells. Phys. Rev. Appl. 2019, 11, 054013. [Google Scholar] [CrossRef]
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407. [Google Scholar] [CrossRef]




| Sample (Dots’ Concentration, Size) | Film Thickness (nm) |
|---|---|
| Pristine MAPbI3 Perovskite (PVK) | 249 ± 7 |
| CQD@PVK (70 mg/mL, 3 nm dots) | 399 ± 27 |
| CQD@PVK (70 mg/mL, 3.5 nm dots) | 394 ± 27 |
| CQD@PVK (155 mg/mL, 3 nm dots) | 419 ± 32 |
| CQD@PVK (155 mg/mL, 3.5 nm dots) | 594 ± 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, G.; Ferreira, G.; Menda, U.D.; Alexandre, M.; Brites, M.J.; Barreiros, M.A.; Jana, S.; Águas, H.; Martins, R.; Fernandes, P.A.; et al. Sub-Bandgap Sensitization of Perovskite Semiconductors via Colloidal Quantum Dots Incorporation. Nanomaterials 2023, 13, 2447. https://doi.org/10.3390/nano13172447
Ribeiro G, Ferreira G, Menda UD, Alexandre M, Brites MJ, Barreiros MA, Jana S, Águas H, Martins R, Fernandes PA, et al. Sub-Bandgap Sensitization of Perovskite Semiconductors via Colloidal Quantum Dots Incorporation. Nanomaterials. 2023; 13(17):2447. https://doi.org/10.3390/nano13172447
Chicago/Turabian StyleRibeiro, G., G. Ferreira, U. D. Menda, M. Alexandre, M. J. Brites, M. A. Barreiros, S. Jana, H. Águas, R. Martins, P. A. Fernandes, and et al. 2023. "Sub-Bandgap Sensitization of Perovskite Semiconductors via Colloidal Quantum Dots Incorporation" Nanomaterials 13, no. 17: 2447. https://doi.org/10.3390/nano13172447