Bismuth Tungstate Nanoplates—Vis Responsive Photocatalyst for Water Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterization of Photocatalyst
2.4. Photocatalytic Activity Tests
3. Results
3.1. Characterization
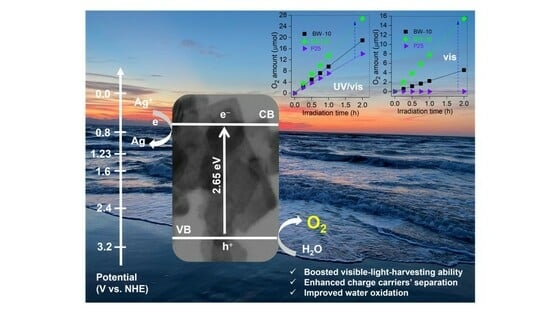
3.2. Photocatalytic O2 Evolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayat, A.; Sohail, M.; Taha, T.A.; Kumar Baburao Mane, S.; Al-Sehemi, A.G.; Al-Ghamdi, A.A.; Nawawi, W.I.; Palamanit, A.; Amin, M.A.; Fallatah, A.M.; et al. Synergetic effect of bismuth vanadate over copolymerized carbon nitride composites for highly efficient photocatalytic H2 and O2 generation. J. Colloid Interface Sci. 2022, 627, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Djatoubai, E.; Khan, M.S.; ul Haq, S.; Guo, P.; Shen, S. BiFeO3 bandgap engineering by dopants and defects control for efficient photocatalytic water oxidation. Appl. Catal. A-Gen. 2022, 643, 118737. [Google Scholar] [CrossRef]
- Ma, K.; Yang, B.; Su, X.; Fan, L. Two-phase solvothermal preparation of Co3O4/GO compound materials as catalysts for photocatalytic water oxidation. Mater. Lett. 2022, 324, 132615. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, L.; Konysheva, E.Y.; Xu, X. Expediting photocarrier separation in Ta3N5@CaTaO2N heterostructures with seamless interfaces for photocatalytic water oxidation under visible light. Appl. Catal. B 2022, 317, 121712. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Ke, G.; Liu, B.; Dong, F.; Yang, L.; He, H.; Zhou, Y. WO3 homojunction photoanode: Integrating the advantages of WO3 different facets for efficient water oxidation. J. Energy Chem. 2021, 56, 37–45. [Google Scholar] [CrossRef]
- Chen, Z.; Geng, S.; Wang, Y.; Wang, Y.; Song, S. Boosting 2 e-Water oxidation reaction on WO3 by F-modification and revealing the mechanism by probing interfacial water structure. Appl. Catal. B 2022, 317, 121756. [Google Scholar] [CrossRef]
- Sun, W.; Dong, Y.; Zhai, X.; Zhang, M.; Li, K.; Wang, Q.; Ding, Y. Crystal facet engineering of BiVO4/CQDs/TPP with improved charge transfer efficiency for photocatalytic water oxidation. Chem. Eng. J. 2022, 430, 132872. [Google Scholar] [CrossRef]
- Ke, G.; Liu, B.; Duan, F.; Liu, X.; Wen, J.; Jia, B.; Liu, X.; He, H.; Zhou, Y. Resorcinol-formaldehyde resin nanoparticles as surface charge transfer and separation sites for the improvement of BiVO4 film photoanodes’ performance in solar water oxidation. Appl. Surf. Sci. 2022, 601, 154236. [Google Scholar] [CrossRef]
- Khan, N.A.; Rashid, N.; Ahmad, I.; Zahidullah; Zairov, R.; ur Rehman, H.; Nazar, M.F.; Jabeen, U. An efficient Fe2O3/FeS heterostructures water oxidation catalyst. Int. J. Hydrog. Energy 2022, 47, 22340–22347. [Google Scholar] [CrossRef]
- Duc Quang, N.; Cao Van, P.; Majumder, S.; Jeong, J.-R.; Kim, D.; Kim, C. Rational construction of S-doped FeOOH onto Fe2O3 nanorods for enhanced water oxidation. J. Colloid Interface Sci. 2022, 616, 749–758. [Google Scholar] [CrossRef]
- Zhu, Z.; Wan, S.; Zhao, Y.; Qin, Y.; Ge, X.; Zhong, Q.; Bu, Y. Recent progress in Bi2WO6-Based photocatalysts for clean energy and environmental remediation: Competitiveness, challenges, and future perspectives. Nano Sel. 2021, 2, 187–215. [Google Scholar] [CrossRef]
- Khedr, T.M.; Wang, K.; Kowalski, D.; El-Sheikh, S.M.; Abdeldayem, H.M.; Ohtani, B.; Kowalska, E. Bi2WO6-based Z-scheme photocatalysts: Principles, mechanisms and photocatalytic applications. J. Environ. Chem. Eng. 2022, 10, 107838. [Google Scholar] [CrossRef]
- Kudo, A.; Hijii, S. H2 or O2 evolution from aqueous solutions on layered oxide photocatalysts consisting of Bi3+ with 6s2 configuration and d0 transition metal ions. Chem. Lett. 1999, 28, 1103–1104. [Google Scholar] [CrossRef]
- Guo, W.; Jian, L.; Wang, X.; Zeng, W. Hydrothermal synthesis of Ni-doped hydrangea-like Bi2WO6 and the enhanced gas sensing property to n-butanol. Sens. Actuators B Chem. 2022, 357, 131396. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Y.; Yi, C.; Xu, J.; Wang, B.; Ma, R.; Xu, M. Construction of carbon dots modified Cl-doped Bi2WO6 hollow microspheres for boosting photocatalytic degradation of tetracycline under visible light irradiation. Ceram. Int. 2022, 49, 7214–7222. [Google Scholar] [CrossRef]
- Bera, S.; Samajdar, S.; Pal, S.; Das, P.S.; Jones, L.A.H.; Finch, H.; Dhanak, V.R.; Ghosh, S. Effect of metal doping in Bi2WO6 micro-flowers for enhanced photoelectrochemical water splitting. Ceram. Int. 2022, 48, 35814–35824. [Google Scholar] [CrossRef]
- Su, H.; Li, S.; Xu, L.; Liu, C.; Zhang, R.; Tan, W. Hydrothermal preparation of flower-like Ni2+ doped Bi2WO6 for enhanced photocatalytic degradation. J. Phys. Chem. Solids 2022, 170, 110954. [Google Scholar] [CrossRef]
- Alhadi, A.; Ma, S. Synthesis of Sn doped-Bi2WO6 nanoslices for enhanced isopropanol sensing properties. Phys. B Condens. Matter 2022, 635, 413819. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Buapoon, S.; Bunluesak, T.; Suebsom, P.; Wannapop, S.; Thongtem, T.; Thongtem, S. Hydrothermal preparation of Au-doped Bi2WO6 nanoplates for enhanced visible-light-driven photocatalytic degradation of rhodamine B. Solid State Sci. 2022, 128, 106881. [Google Scholar] [CrossRef]
- Ai, S.; Liu, Y.; Chai, Y.; Yuan, R.; Liu, H. Enhanced cathodic photocurrent derived from N-type S doped-Bi2WO6 nanoparticles through an antenna-like strategy for photoelectrochemical biosensor. Biosens. Bioelectron. 2022, 207, 114176. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, J.; Dai, R.; Wu, Q.; Zhang, L.; Zhang, W.; Yan, J.; Zhang, F. Experiment and DFT study on the photocatalytic properties of La-doped Bi2WO6 nanoplate-like materials. Appl. Surf. Sci. 2022, 579, 152219. [Google Scholar] [CrossRef]
- Sun, D.; Le, Y.; Jiang, C.; Cheng, B. Ultrathin Bi2WO6 nanosheet decorated with Pt nanoparticles for efficient formaldehyde removal at room temperature. Appl. Surf. Sci. 2018, 441, 429–437. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, H.; Xu, Q.; Song, X.; Zhai, C.; Zhu, M. Pt decorated 2D/3D heterostructure of Bi2WO6 nanosheet/Cu2S snowflake for improving electrocatalytic methanol oxidation with visible-light assistance. Appl. Surf. Sci. 2020, 521, 146431. [Google Scholar] [CrossRef]
- Jin, K.; Qin, M.; Li, X.; Wang, R.; Zhao, Y.; Li, Y.; Wang, H. A low-dosage silver-loaded flower-like Bi2WO6 nanosheets toward efficiently photocatalytic degradation of sulfamethoxazole. Mater. Sci. Semicond. Process. 2022, 139, 106338. [Google Scholar] [CrossRef]
- Li, Z.; Wang, K.; Zhang, J.; Chang, Y.; Kowalska, E.; Wei, Z. Enhanced Photocatalytic Activity of Hierarchical Bi2WO6 Microballs by Modification with Noble Metals. Catalysts 2022, 12, 130. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Ge, Y.; Li, H.; Ji, H.; Xu, H.; Zhang, Q.; Li, H.; Li, M. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl. Catal. B 2015, 168–169, 51–61. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Deng, Y.; Dong, H.; Liu, Y.; Wang, L.; Peng, B.; Zhang, C.; Chen, F. 0D/2D interface engineering of carbon quantum dots modified Bi2WO6 ultrathin nanosheets with enhanced photoactivity for full spectrum light utilization and mechanism insight. Appl. Catal. B 2018, 222, 115–123. [Google Scholar] [CrossRef]
- Shad, N.A.; Sajid, M.M.; Afzal, A.M.; Amin, N.; Javed, Y.; Hassan, S.; Imran, Z.; Razaq, A.; Yousaf, M.I.; Munawar, A.; et al. Facile synthesis of Bi2WO6/rGO nanocomposites for photocatalytic and solar cell applications. Ceram. Int. 2021, 47, 16101–16110. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, J.; Zang, S.; Gong, L.; Feng, L. Constructing broad spectrum response ROQDs/Bi2WO6/CQDs heterojunction nanoplates: Synergetic mechanism of boosting redox abilities for photocatalytic degradation pollutant. J. Environ. Chem. Eng. 2021, 9, 105674. [Google Scholar] [CrossRef]
- Madani, M.; Mansourian, M.; Almadari, S.; Mirzaee, O.; Tafreshi, M.J. Enhanced photosensitivity of heterostructure SiO2/Bi2WO6/GO composite nanoparticles. Phys. B Condens. Matter 2022, 645, 414241. [Google Scholar] [CrossRef]
- Riaz, A.; Saeed, M.; Munir, M.; Intisar, A.; Haider, S.; Tariq, S.; Hussain, N.; Kousar, R.; Bilal, M. Development of reduced graphene oxide-supported novel hybrid nanomaterials (Bi2WO6@rGO and Cu-WO4@rGO) for green and efficient oxidative desulfurization of model fuel oil for environmental depollution. Environ. Res. 2022, 212, 113160. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, B.; Fan, X.; Wang, W.; Chen, X.; Shao, N.; Jiang, P. Ag/rGO/Bi2WO6 nanocomposite as a highly efficient and stable photocatalyst for rhodamine B degradation under visible light irradiation. Diam. Relat. Mater. 2022, 127, 109143. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Y. Synthesis of Square Bi2WO6 Nanoplates as High-Activity Visible-Light-Driven Photocatalysts. Chem. Mater. 2005, 17, 3537–3545. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Huang, X. Synthesis and Visible-Light Photocatalytic Property of Bi2WO6 Hierarchical Octahedron-Like Structures. Nanoscale Res. Lett. 2008, 3, 365–371. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, L.; Xie, Y.; Lin, Z.; Fan, Y.; Liu, D.; Chen, L.; Zhang, Z.; Wang, X. Controllable synthesis of Bi2WO6 nanoplate self-assembled hierarchical erythrocyte microspheres via a one-pot hydrothermal reaction with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 403, 326–334. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Zhou, L.; Xu, H. Bi2WO6 nano-and microstructures: Shape control and associated visible-light-driven photocatalytic activities. Small 2007, 3, 1618–1625. [Google Scholar] [CrossRef]
- Yang, P.; Chen, C.; Wang, D.; Ma, H.; Du, Y.; Cai, D.; Zhang, X.; Wu, Z. Kinetics, reaction pathways, and mechanism investigation for improved environmental remediation by 0D/3D CdTe/Bi2WO6 Z-scheme catalyst. Appl. Catal. B 2021, 285, 119877. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Somaudon, V.; Photiwat, T.; Hemavibool, K.; Nanan, S. Preparation, characterization, and photocatalytic study of solvothermally grown CTAB-capped Bi2WO6 photocatalyst toward photodegradation of rhodamine B dye. Opt. Mater. 2021, 117, 111183. [Google Scholar] [CrossRef]
- Wu, L.; Zheng, S.; Lin, H.; Zhou, S.; Mahmoud Idris, A.; Wang, J.; Li, S.; Li, Z. In-situ assembling 0D/2D Z-scheme heterojunction of lead-free Cs2AgBiBr6/Bi2WO6 for enhanced photocatalytic CO2 reduction. J. Colloid Interface Sci. 2022, 629, 233–242. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, J.; Chen, C.; Yang, S.; Lin, J.; Xi, J.; Kong, Z. Novel 0D/2D Bi2WO6/MoSSe Z-scheme heterojunction for enhanced photocatalytic degradation and photoelectrochemical activity. Ceram. Int. 2022, 48, 31970–31983. [Google Scholar] [CrossRef]
- Jin, K.; Qin, M.; Li, X.; Wang, R.; Zhao, Y.; Wang, H. Z-scheme Au@TiO2/Bi2WO6 heterojunction as efficient visible-light photocatalyst for degradation of antibiotics. J. Mol. Liq. 2022, 364, 120017. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, J.; Zeng, K. Synthesis of Bi2WO6/g-C3N4 heterojunction on activated carbon fiber membrane as a thin-film photocatalyst for treating antibiotic wastewater. Inorg. Chem. Commun. 2022, 140, 109418. [Google Scholar] [CrossRef]
- Atla, R.; Oh, T.H. Novel fabrication of the recyclable MoS2/Bi2WO6 heterostructure and its effective photocatalytic degradation of tetracycline under visible light irradiation. Chemosphere 2022, 303, 134922. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Y.; Wang, J.; Liu, S.; Chang, Y.; Xu, X.; Feng, C.; Xu, J.; Guo, L.; Xu, J.; et al. Photo-Fenton and oxygen vacancies’ synergy for enhancing catalytic activity with S-scheme FeS2/Bi2WO6 heterostructure. Catal. Sci. Technol. 2022, 12, 4228–4242. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Li, Z.; Sun, J.; Yang, J.; Wei, J.; Wang, S.; Song, H.; Hou, Y. Visible light driven antibiotics degradation using S-scheme Bi2WO6/CoIn2S4 heterojunction: Mechanism, degradation pathways and toxicity assessment. Chemosphere 2022, 303, 135113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Duan, J.; Hou, B. An S-scheme heterojunction of Bi2WO6/AgIO3 nanocomposites that enhances photocatalytic degradation of rhodamine B and antifouling properties. Ceram. Int. 2022, 48, 24777–24787. [Google Scholar] [CrossRef]
- Rabanimehr, F.; Farhadian, M.; Nazar, A.R.S. A high-performance microreactor integrated with chitosan/Bi2WO6/CNT/TiO2 nanofibers for adsorptive/photocatalytic removal of cephalexin from aqueous solution. Int. J. Biol. Macromol. 2022, 208, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Sattari, M.; Farhadian, M.; Reza Solaimany Nazar, A.; Moghadam, M. Enhancement of Phenol degradation, using of novel Z-scheme Bi2WO6/C3N4/TiO2 composite: Catalyst and operational parameters optimization. J. Photochem. Photobiol. A 2022, 431, 114065. [Google Scholar] [CrossRef]
- Chu, Y.; Fan, J.; Wang, R.; Liu, C.; Zheng, X. Preparation and immobilization of Bi2WO6/BiOI/g-C3N4 nanoparticles for the photocatalytic degradation of tetracycline and municipal waste transfer station leachate. Sep. Purif. Technol. 2022, 300, 121867. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Su, M.; Sun, H.; Tian, Z.; Zhao, Z.; Li, P. Z-scheme 2D/2D WS2/Bi2WO6 heterostructures with enhanced photocatalytic performance. Appl. Catal. A-Gen. 2022, 631, 118485. [Google Scholar] [CrossRef]
- Liang, L.; Lei, F.; Gao, S.; Sun, Y.; Jiao, X.; Wu, J.; Qamar, S.; Xie, Y. Single unit cell bismuth tungstate layers realizing robust solar CO2 reduction to methanol. Angew. Chemie-Int. Ed. 2015, 54, 13971–13974. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lin, M.; Long, J.; Zhang, Z.; Lin, H.; Wu, J.C.-S.; Wang, X. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 2015, 6, 8340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hua, X.; Teng, X.; Liu, D.; Qin, Z.; Ding, S. CTAB assisted hydrothermal synthesis of lamellar Bi2WO6 with superior photocatalytic activity for rhodamine B degradation. Mater. Lett. 2016, 185, 275–277. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, P.; Meng, X.; Tang, Y.; Huang, P.; Chen, X.; Shen, X.; Zeng, X. CTAB-assisted fabrication of Bi2WO6 thin nanoplates with high adsorption and enhanced visible light-driven photocatalytic performance. Molecules 2017, 22, 859. [Google Scholar] [CrossRef]
- Jakhade, A.P.; Biware, M.V.; Chikate, R.C. Two-dimensional Bi2WO6 nanosheets as a robust catalyst toward photocyclization. ACS Omega 2017, 2, 7219–7229. [Google Scholar] [CrossRef]
- Wu, S.; Sun, J.; Li, Q.; Hood, Z.D.; Yang, S.; Su, T.; Peng, R.; Wu, Z.; Sun, W.; Kent, P.R.C.; et al. Effects of Surface Terminations of 2D Bi2WO6 on Photocatalytic Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 20067–20074. [Google Scholar] [CrossRef]
- Saovakon, C.; Jansanthea, P. Inhibition of pathogenic bacteria by using cupric oxide nanostructures synthesized via CTAB-assisted hydrothermal route. J. Aust. Ceram. Soc. 2020, 56, 1385–1396. [Google Scholar] [CrossRef]
- Leandro, M.K.; Moura, J.V.; Freire, P.D.; Vega, M.L.; Lima, C.D.; Hidalgo, Á.A.; Araújo, A.C.; Freitas, P.R.; Paulo, C.L.; Sousa, A.K.; et al. Characterization and evaluation of layered Bi2WO6 nanosheets as a new antibacterial agent. Antibiotics 2021, 10, 1068. [Google Scholar] [CrossRef]
- Pang, B.; Liu, S.; Tu, Y.; Wang, X. Controllable synthesis and enhanced photoactivity of two-dimensional Bi2WO6 ultra-thin nanosheets. ChemistrySelect 2021, 6, 5381–5386. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Hu, J.; Li, Z. Synthesis and characterization of Bi2WO6 nanoplates using egg white as a biotemplate through sol-gel method. Mater. Lett. 2015, 139, 401–404. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, H.; Wang, B.; Li, R.; Wang, X. Effect of experimental parameters on the hydrothermal synthesis of Bi2WO6 nanostructures. Nanoscale. Res. Lett. 2016, 11, 190. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, X.; Tong, L.; Zeng, X.; Chen, X. Template-free fabrication of Bi2WO6 hierarchical hollow microspheres with visible-light-driven photocatalytic activity. Energies 2016, 9, 764. [Google Scholar] [CrossRef]
- Xie, H.; Shen, D.; Wang, X.; Shen, G. Microwave hydrothermal synthesis and visible-light photocatalytic activity of Bi2WO6 nanoplates. Mater. Chem. Phys. 2007, 103, 334–339. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Dumrongrojthanath, P.; Ekthammathat, N.; Thongtem, S.; Thongtem, T. Hydrothermal synthesis, characterization, and visible light-driven photocatalytic properties of Bi2WO6 nanoplates. J. Nanomater. 2014, 2014, 138561. [Google Scholar] [CrossRef]
- Nagyné-Kovács, T.; Shahnazarova, G.; Lukács, I.E.; Szabó, A.; Hernadi, K.; Igricz, T.; László, K.; Szilágyi, I.M.; Pokol, G. Effect of pH in the hydrothermal preparation of Bi2WO6 nanostructures. Materials 2019, 12, 1728. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication–hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [PubMed]
- Suwannaruang, T.; Rivera, K.K.P.; Neramittagapong, A.; Wantala, K. Effects of hydrothermal temperature and time on uncalcined TiO2 synthesis for reactive red 120 photocatalytic degradation. Surf. Coat. Technol. 2015, 271, 192–200. [Google Scholar] [CrossRef]
- Zhu, X.; Wen, G.; Liu, H.; Han, S.; Chen, S.; Kong, Q.; Feng, W. One-step hydrothermal synthesis and characterization of Cu-doped TiO2 nanoparticles/nanobucks/nanorods with enhanced photocatalytic performance under simulated solar light. J. Mater. Sci. Mater. Electron. 2019, 30, 13826–13834. [Google Scholar] [CrossRef]
- Tang, M.; Xia, Y.; Yang, D.; Liu, J.; Zhu, X.; Tang, R. Effects of hydrothermal time on structure and photocatalytic property of titanium dioxide for degradation of rhodamine B and tetracycline Hydrochloride. Materials 2021, 14, 5674. [Google Scholar] [CrossRef]
- Mezzourh, H.; Ben Moumen, S.; Amjoud, M.; Mezzane, D.; El Amraoui, Y.; Marbati, B.; Lahmar, A.; Jouiad, M.; El Marssi, M. Effect of growth time on structural and surface properties of TiO2 nanostructures deposited by single-step hydrothermal method. Mater. Today Proc. 2022, 51, 2053–2058. [Google Scholar] [CrossRef]
- Majid, F.; Bashir, M.; Bibi, I.; Raza, A.; Ezzine, S.; Alwadai, N.; Iqbal, M. ZnO nanofibers fabrication by hydrothermal route and effect of reaction time on dielectric, structural and optical properties. J. Mater. Res. Technol. 2022, 18, 4019–4029. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; Li, J.; Pan, Z.; Ma, H.; Jiang, Y.; Tian, Z. Facile hydrothermal synthesis of MoS2 nano-sheets with controllable structures and enhanced catalytic performance for anthracene hydrogenation. RSC Adv. 2016, 6, 71534–71542. [Google Scholar] [CrossRef]
- Luo, L.; Shi, M.; Zhao, S.; Tan, W.; Lin, X.; Wang, H.; Jiang, F. Hydrothermal synthesis of MoS2 with controllable morphologies and its adsorption properties for bisphenol A. J. Saudi Chem. Soc. 2019, 23, 762–773. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Nie, T.; Wang, R.; He, B.; Han, B.; Wang, H.; Tian, Y.; Gong, Y. Enhanced visible-light photocatalytic H2 production of hierarchical g-C3N4 hexagon by one-step self-assembly strategy. Appl. Surf. Sci. 2020, 499, 143942. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhang, L.; Gao, E.; Jiang, D.; Sun, Y.; Xie, Y. Ultrathin {001}-oriented bismuth tungsten oxide nanosheets as highly efficient photocatalysts. ChemSusChem 2013, 6, 1873–1877. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Tang, L.; Zeng, G.; Wang, J.; Zhu, Y.; Feng, C.; Deng, Y.; He, W. Ultrathin Bi2WO6 nanosheets loaded g-C3N4 quantum dots: A direct Z-scheme photocatalyst with enhanced photocatalytic activity towards degradation of organic pollutants under wide spectrum light irradiation. J. Colloid Interface Sci. 2019, 539, 654–664. [Google Scholar] [CrossRef]
- Marotti, R.E.; Giorgi, P.; Machado, G.; Dalchiele, E.A. Crystallite size dependence of band gap energy for electrodeposited ZnO grown at different temperatures. Sol. Energy Mater. Sol. Cells 2006, 90, 2356–2361. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef]
- Singh, M.; Taele, B.M.; Goyal, M. Modeling of size and shape dependent band gap, dielectric constant and phonon frequency of semiconductor nanosolids. Chin. J. Phys. 2021, 70, 26–36. [Google Scholar] [CrossRef]
- Djatoubai, E.; Khan, M.S.; ul Haq, S.; Guo, P.; Shen, S. Rational design of BiFeO3 nanostructures for efficient charge carrier transfer and consumption for photocatalytic water oxidation. J. Alloys Compd. 2022, 911, 164920. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.-O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Wang, K.; Wei, Z.; Colbeau-Justin, C.; Nitta, A.; Kowalska, E. P25 and its components—Electronic properties and photocatalytic activities. Surf. Interfaces 2022, 31, 102057. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Tanaka, M.; Ohtani, B. Correlation between surface area and photocatalytic activity for acetaldehyde decomposition over bismuth tungstate particles with a hierarchical structure. Langmuir 2010, 26, 7174–7180. [Google Scholar] [CrossRef]
- Liu, B.; Nakata, K.; Sakai, M.; Saito, H.; Ochiai, T.; Murakami, T.; Takagi, K.; Fujishima, A. Hierarchical TiO2 spherical nanostructures with tunable pore size, pore volume, and specific surface area: Facile preparation and high-photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1933–1939. [Google Scholar] [CrossRef]
- Mohtar, S.S.; Aziz, F.; Ismail, A.F.; Sambudi, N.S.; Abdullah, H.; Rosli, A.N.; Ohtani, B. Impact of doping and additive applications on photocatalyst textural properties in removing organic pollutants: A review. Catalysts 2021, 11, 1160. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Endo-Kimura, M.; Wang, K.; Ohtani, B.; Kowalska, E. Development of sulfur-doped graphitic carbon nitride for hydrogen evolution under visible-light irradiation. Nanomaterials 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Faycal Atitar, M.; Ismail, A.A.; Al-Sayari, S.A.; Bahnemann, D.; Afanasev, D.; Emeline, A. V Mesoporous TiO2 nanocrystals as efficient photocatalysts: Impact of calcination temperature and phase transformation on photocatalytic performance. Chem. Eng. J. 2015, 264, 417–424. [Google Scholar] [CrossRef]
- Fao, G.D.; Catherine, H.N.; Huang, C.H.; Lee, Y.L.; Jiang, J.C.; Hu, C. Unraveling the effects of P and S doping over g-C3N4 in strengthening Lewis basicity for CO2/glycerol conversion: A theoretical and experimental study. Carbon 2023, 201, 129–140. [Google Scholar] [CrossRef]
- Alzahrani, K.A.; Ismail, A.A. α-Fe2O3/CeO2 S-scheme heterojunction photocatalyst for enhanced photocatalytic H2 evolution. Surf. Interfaces 2023, 39, 102935. [Google Scholar] [CrossRef]
- Alzahrani, K.A.; Ismail, A.A. Highly efficient AgVO3/WO3 photocatalyst n-n heterojunction toward visible-light induced degradation antibiotic. J. Ind. Eng. Chem. 2023, 124, 270–278. [Google Scholar] [CrossRef]
- Wang, X.; Sø, L.; Su, R.; Wendt, S.; Hald, P.; Mamakhel, A.; Yang, C.; Huang, Y.; Iversen, B.B.; Besenbacher, F. The influence of crystallite size and crystallinity of anatase nanoparticles on the photo-degradation of phenol. J. Catal. 2014, 310, 100–108. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Zaen, R.; Oktiani, R. Correlation between Crystallite Size and Photocatalytic Performance of Micrometer-Sized Monoclinic WO3 Particles. Arab. J. Chem. 2020, 13, 1283–1296. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.V.; Hisanaga, T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, Y.; Nishimoto, S.I. Photocatalytic activity of amorphous-anatase mixture of titanium(iv) oxide particles suspended in aqueous solutions. J. Phys. Chem. B 1997, 101, 3746–3752. [Google Scholar] [CrossRef]
- Peng, T.; Zhao, D.; Dai, K.; Shi, W.; Hirao, K. Synthesis of titanium dioxide nanoparticles with mesoporous anatase wall and high photocatalytic activity. J. Phys. Chem. B 2005, 109, 4947–4952. [Google Scholar] [CrossRef]
- Arias, L.M.F.; Duran, A.A.; Cardona, D.; Camps, E.; Gómez, M.E.; Zambrano, G. Effect of annealing treatment on the photocatalytic activity of TiO2 thin films deposited by dc reactive magnetron sputtering. J. Phys. Conf. Ser. 2015, 614, 012008. [Google Scholar] [CrossRef]
- Vamvasakis, I.; Georgaki, I.; Vernardou, D.; Kenanakis, G.; Katsarakis, N. Synthesis of WO3 catalytic powders: Evaluation of photocatalytic activity under NUV/visible light irradiation and alkaline reaction pH. J. Sol-Gel Sci. Technol. 2015, 76, 120–128. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, L.; Guo, J. Effects of calcination on the photocatalytic properties of nanosized TiO2 powders prepared by TiCl4 hydrolysis. Appl. Catal. B Environ. 2000, 26, 207–215. [Google Scholar] [CrossRef]
- Liu, S.; Jaffrezic, N.; Guillard, C. Size effects in liquid-phase photo-oxidation of phenol using nanometer-sized TiO2 catalysts. Appl. Surf. Sci. 2008, 255, 2704–2709. [Google Scholar] [CrossRef]
- Murakami, N.; Kawakami, S.; Tsubota, T.; Ohno, T. Dependence of photocatalytic activity on particle size of a shape-controlled anatase titanium(IV) oxide nanocrystal. J. Mol. Catal. A Chem. 2012, 358, 106–111. [Google Scholar] [CrossRef]
- Rasalingam, S.; Wu, C.M.; Koodali, R.T. Modulation of pore sizes of titanium dioxide photocatalysts by a facile template free hydrothermal synthesis method: Implications for photocatalytic degradation of Rhodamine B. ACS Appl. Mater. Interfaces 2015, 7, 4368–4380. [Google Scholar] [CrossRef]
- Chandran, N.; Jayakrishnan, R.; Abraham, R. Role of pore size on the photocatalytic dilapidation of organic pollutant SRB in mesoporous In2S3. J. Chem. Sci. 2023, 135, 78. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Wang, Z.; Gao, P.; Li, G.K. Synthesis and application of mesoporous materials: Process status, technical problems, and development prospects: A mini-review. Energy Fuels 2023, 37, 3413–3427. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Ohtani, B.; Kowalska, E. Photonic crystals for plasmonic photocatalysis. Catalysts 2020, 10, 827. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Kowalska, E.; Abdeldayem, H.M. The synergistic effect of anatase and brookite for photocatalytic generation of hydrogen and diclofenac degradation. J. Environ. Chem. Eng. 2021, 9, 106566. [Google Scholar] [CrossRef]
- Tian, L.; Xian, X.; Cui, X.; Tang, H.; Yang, X. Fabrication of modified g-C3N4 nanorod/Ag3PO4 nanocomposites for solar-driven photocatalytic oxygen evolution from water splitting. Appl. Surf. Sci. 2018, 430, 301–308. [Google Scholar] [CrossRef]
- Tian, L.; Yang, X.; Cui, X.; Liu, Q.; Tang, H. Fabrication of dual direct Z-scheme g-C3N4/MoS2/Ag3PO4 photocatalyst and its oxygen evolution performance. Appl. Surf. Sci. 2019, 463, 9–17. [Google Scholar] [CrossRef]
- Zhou, H.; Ke, J.; Xu, D.; Liu, J. MnWO4 nanorods embedded into amorphous MoSx microsheets in 2D/1D MoSx/MnWO4 S–scheme heterojunction for visible-light photocatalytic water oxidation. J. Mater. Sci. Technol. 2023, 136, 169–179. [Google Scholar] [CrossRef]
- Tang, Q.-Y.; Chen, W.-F.; Lv, Y.-R.; Yang, S.-Y.; Xu, Y.-H. Z-scheme hierarchical Cu2S/Bi2WO6 composites for improved photocatalytic activity of glyphosate degradation under visible light irradiation. Sep. Purif. Technol. 2020, 236, 116243. [Google Scholar] [CrossRef]












| Sample ID | Hydrothermal Time (h) | 1 d (113) (Ǻ) | 2 FWHM (Ǻ) | 3 ACS (nm) | Crystallinity (%) |
|---|---|---|---|---|---|
| BWO–10 | 10 | 3.162 | 1.0371 | 7.8 | 48.2 |
| BWO–15 | 15 | 3.160 | 1.0104 | 8 | 52.9 |
| BWO–20 | 20 | 3.159 | 0.9450 | 8.6 | 56.1 |
| BWO–25 | 25 | 3.157 | 0.7149 | 11.5 | 75.2 |
| BWO–30 | 30 | 3.156 | 0.6847 | 11.8 | 77.8 |
| BWO–40 | 40 | 3.158 | 0.7191 | 11.2 | 74.6 |
| Catalyst ID | 1 SSA/m2 g−1 | 2 PV/cm3 g−1 × 10−4 | 3 PS/nm | 4 AE/nm | 5 Eg/eV |
|---|---|---|---|---|---|
| BWO–10 | 55.3 | 5.8–6.2 | 3.9–9.9 | 433.0 | 2.87 |
| BWO–15 | 51.5 | 5.7–6.0 | 4.3–10.8 | 442.2 | 2.77 |
| BWO–20 | 48.7 | 5.3–5.7 | 4.7–11.7 | 450.0 | 2.75 |
| BWO–25 | 44.8 | 4.5–4.8 | 5.4–13.4 | 460.3 | 2.67 |
| BWO–30 | 43.9 | 4.1–4.4 | 5.5–13.7 | 474.1 | 2.65 |
| BWO–40 | 46.6 | 4.9–5.4 | 5.1–12.8 | 452.8 | 2.70 |
| Catalyst ID | Under UV-Vis Irradiation | Under Vis Irradiation | ||||
|---|---|---|---|---|---|---|
| Evolved O2 Amount/ μ mol | Evolved O2 Rate/ μ mol h−1 | R2 | Evolved O2 Amount/ μ mol | Evolved O2 Rate/ μ mol h−1 | R2 | |
| BWO–10 | 18.99 | 9.54 | 0.9998 | 4.55 | 2.27 | 0.9999 |
| BWO–15 | 20.45 | 10.24 | 0.9999 | 6.78 | 3.40 | 0.9999 |
| BWO–20 | 22.89 | 11.44 | 0.9997 | 9.31 | 4.66 | 0.9999 |
| BWO–25 | 25.87 | 13.00 | 0.9999 | 11.22 | 5.62 | 0.9999 |
| BWO–30 | 26.78 | 13.40 | 0.9999 | 15.45 | 7.74 | 1.0000 |
| BWO–40 | 24.98 | 12.53 | 0.9998 | 12.89 | 6.43 | 0.9999 |
| P25 | 14.13 | 7.10 | 0.9998 | 0.011 | 0.006 | 0.9999 |
| Catalyst/ Dose (g) | Light Source | Reactant Suspension | Irradiation Time (min) | O2 Rate (μmol h−1) | Ref. |
|---|---|---|---|---|---|
| g-C3N4/ Ag3PO4/ 0.3 | white LED light | 100 mL aq. solution (1 g AgNO3) | 60 | 3.30 | [108] |
| g-C3N4/ MoS2 Ag3PO4/ 0.3 | white LED light | 100 mL aq. solution (1 g AgNO3) | 40 | 6.99 | [109] |
| Ti/ BiFeO3/ 0.01 | 300-W Xe lamp, λ > 420 nm; UV cut–off filter: Y–42) | 80 mL aq. solution (0.14 g AgNO3 + 0.16 g La2O3) | 360 | 2.74 | [2] |
| MoS2/ MnWO4/ 0.05 | 300-W Xe lamp, λ > 420 nm; UV cut–off filter: Y–42) | 200 mL aq. solution (0.03 M AgNO3 + 0.2 g La2O3) | 180 | 5.19 | [111] |
| 2D Bi2WO6/ 0.05 | Hg lamp, λ > 290 nm | 5 mL aq. solution (0.05 M AgF) | 120 | 13.40 | This work |
| 300-W Xe lamp, λ > 420 nm; UV cut–off filter: Y–42) | 7.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khedr, T.M.; El-Sheikh, S.M.; Kowalska, E. Bismuth Tungstate Nanoplates—Vis Responsive Photocatalyst for Water Oxidation. Nanomaterials 2023, 13, 2438. https://doi.org/10.3390/nano13172438
Khedr TM, El-Sheikh SM, Kowalska E. Bismuth Tungstate Nanoplates—Vis Responsive Photocatalyst for Water Oxidation. Nanomaterials. 2023; 13(17):2438. https://doi.org/10.3390/nano13172438
Chicago/Turabian StyleKhedr, Tamer M., Said M. El-Sheikh, and Ewa Kowalska. 2023. "Bismuth Tungstate Nanoplates—Vis Responsive Photocatalyst for Water Oxidation" Nanomaterials 13, no. 17: 2438. https://doi.org/10.3390/nano13172438