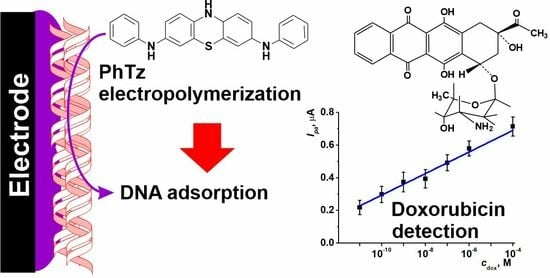
Voltammetric Sensor for Doxorubicin Determination Based on Self-Assembled DNA-Polyphenothiazine Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Electrode Modification
3. Results and Discussion
3.1. PhTz Electropolymerization
3.2. DNA Immobilization
3.2.1. Voltammetric Study
3.2.2. EIS Study
3.2.3. SEM Study
3.3. Measurement Conditions
3.3.1. Cyclic Voltammetry
3.3.2. Measurement Precision and Sensor Lifetime
3.3.3. Selectivity and Real Sample Assay
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Model List of Essential Medicines: 21st List 2019; World Health Organization: Geneva, Switzerland, 2019.
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Berthiaume, J.M.; Wallace, K.B. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol. Toxicol. 2007, 23, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Kondori, T.; Tajik, S.; Akbarzadeh, T.N.; Beitollahi, H.; Graiff, C. Screen-printed electrode modified with co-NPs, as an electrochemical sensor for simultaneous determination of doxorubicin and dasatinib. J. Iran. Chem. Soc. 2022, 19, 4423–4434. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, R.; Gu, S.; Zhang, L.; Li, N.; Qiao, J. Nitrogen and phosphorus co-doped carbon dots as an effective fluorescence probe for the detection of doxorubicin and cell imaging. Opt. Mater. 2022, 128, 112323. [Google Scholar] [CrossRef]
- Del Bonis-O’Donnell, J.T.; Pinals, R.L.; Jeong, S.; Thakrar, A.; Wolfinger, R.D.; Landry, M.P. Chemometric approaches for developing infrared nanosensors to image anthracyclines. Biochemistry 2019, 58, 54–64. [Google Scholar] [CrossRef]
- Fleury-Souverain, S.; Maurin, J.; Guillarme, D.; Rudaz, S.; Bonnabry, P. Development and application of a liquid chromatography coupled to mass spectrometry method for the simultaneous determination of 23 antineoplastic drugs at trace levels. J. Pharm. Biomed. Anal. 2022, 221, 115034. [Google Scholar] [CrossRef]
- Haq, N.; Alanazi, F.K.; Salem-Bekhit, M.M.; Rabea, S.; Alam, P.; Alsarra, I.A.; Shakeel, F. Greenness estimation of chromatographic assay for the determination of anthracycline-based antitumor drug in bacterial ghost matrix of salmonella typhimurium. Sustain. Chem. Pharm. 2022, 26, 100642. [Google Scholar] [CrossRef]
- Estakhri, N.M.; Edwards, B.; Engheta, N. Inverse-designed metastructures that solve equations. Science 2019, 363, 1333–1338. [Google Scholar] [CrossRef]
- Lalegani, Z.; Ebrahimi, S.A.S.; Hamawandi, B.; La Spada, L.; Batili, H.; Toprak, M.S. Targeted dielectric coating of silver nanoparticles with silica to manipulate optical properties for metasurface applications. Mater. Chem. Phys. 2022, 287, 126250. [Google Scholar] [CrossRef]
- Lalegani, Z.; Ebrahimi, S.A.S.; Hamawandi, B.; La Spada, L.; Toprak, M.S. Modeling, design, and synthesis of gram-scale monodispersed silver nanoparticles using microwave-assisted polyol process for metamaterial applications. Opt. Mater. 2020, 108, 110381. [Google Scholar] [CrossRef]
- Pacheco-Peña, V.; Beruete, M.; Rodríguez-Ulibarri, P.; Engheta, N. On the performance of an ENZ-based sensor using transmission line theory and effective medium approach. New J. Phys. 2019, 21, 043056. [Google Scholar] [CrossRef]
- Akbari, M.; Shahbazzadeh, M.J.; La Spada, L.; Khajehzadeh, A. The graphene field effect transistor modeling based on an optimized ambipolar virtual source model for DNA detection. Appl. Sci. 2021, 11, 8114. [Google Scholar] [CrossRef]
- Zhao, H.; Shi, K.; Zhang, C.; Ren, J.; Cui, M.; Li, N.; Ji, X.; Wang, R. Spherical COFs decorated with gold nanoparticles and multiwalled carbon nanotubes as signal amplifier for sensitive electrochemical detection of doxorubicin. Microchem. J. 2022, 182, 107865. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Khoshnavaz, Y.; Karimi, F.; Çakar, S.; Özacar, M.; Erk, N. A novel 2-dimensional nanocomposite as a mediator for the determination of doxorubicin in biological samples. Environ. Res. 2022, 213, 113590. [Google Scholar] [CrossRef]
- Cunha, C.E.P.; Rodrigues, E.S.B.; De Oliveira Neto, J.R.; Somerset, V.; Taveira, S.; Sgobbi, L.; De Souza Gil, E. Voltammetric glassy carbon sensor approach for the extended stability studies of doxorubicin in lyophilized dosage form. Eclet. Quim. 2022, 47, 32–38. [Google Scholar] [CrossRef]
- Berneth, H.; Bayer, A.G. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Press: Baden-Wurttemberg, Germany, 2003; 585p. [Google Scholar] [CrossRef]
- Brown, J.S. Treatment of cancer with antipsychotic medications: Pushing the boundaries of schizophrenia and cancer. Neurosci. Biobehav. Rev. 2022, 141, 104809. [Google Scholar] [CrossRef]
- Mitchell, S.C. Phenothiazine: The parent molecule. Curr. Drug Targets 2006, 7, 1181–1189. [Google Scholar] [CrossRef]
- Stanković, D.; Dimitrijević, T.; Kuzmanović, D.; Krstić, M.P.; Petković, B.B. Voltammetric determination of an antipsychotic agent trifluoperazine at a boron-doped diamond electrode in human urine. RSC Adv. 2015, 5, 107058–107063. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Sakthivel, R.; Chen, S.-M. Rare earth dysprosium nickelate nanospheres for the selective electrochemical detection of antipsychotic drug perphenazine in biological samples. Mater. Today Chem. 2022, 24, 100883. [Google Scholar] [CrossRef]
- Pulikkutty, S.; Manjula, N.; Chen, T.-W.; Chen, S.-M.; Lou, B.-S.; Siddiqui, M.R.; Wabaidur, S.M.; Ali, M.A. Fabrication of gadolinium zinc oxide anchored with functionalized-SWCNT planted on glassy carbon electrode: Potential detection of psychotropic drug (phenothiazine) in biotic sample. J. Electroanal. Chem. 2022, 918, 116521. [Google Scholar] [CrossRef]
- Abad-Gil, L.; Brett, C.M.A. Poly(methylene blue)-ternary deep eutectic solvent/Au nanoparticle modified electrodes as novel electrochemical sensors: Optimization, characterization and application. Electrochim. Acta 2022, 434, 1141295. [Google Scholar] [CrossRef]
- Manusha, P.; Yadav, S.; Satija, J.; Senthilkumar, S. Designing electrochemical NADH sensor using silver nanoparticles/phenothiazine nanohybrid and investigation on the shape dependent sensing behavior. Sens. Actuators B 2021, 347, 130649. [Google Scholar] [CrossRef]
- Sree, V.G.; Sohn, J.I.; Im, H. Pre-anodized graphite pencil electrode coated with a poly(thionine) film for simultaneous sensing of 3-nitrophenol and 4-nitrophenol in environmental water samples. Sensors 2022, 22, 1151. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Meng, S.; Dong, N.; Liu, C.; Wei, Y.; You, T. Signal-enhanced strategy for ratiometric aptasensing of aflatoxin B1: Plasmon-modulated competition between photoelectrochemistry-driven and electrochemistry-driven redox of methylene blue. Biosens. Bioelectron. 2022, 218, 114759. [Google Scholar] [CrossRef]
- Goida, A.; Kuzin, Y.; Evtugyn, V.; Porfireva, A.; Evtugyn, G.; Hianik, T. Electrochemical sensing of idarubicin—DNA interaction using electropolymerized azure B and methylene blue mediation. Chemosensors 2022, 10, 33. [Google Scholar] [CrossRef]
- Ivanov, A.; Stoikov, D.; Shafigullina, I.; Shurpik, D.; Stoikov, I.; Evtugyn, G. Flow-through acetylcholinesterase sensor with replaceable enzyme reactor. Biosensors 2022, 12, 676. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef]
- Porfireva, A.; Plastinina, K.; Evtugyn, V.; Kuzin, Y.; Evtugyn, G. Electrochemical DNA sensor based on poly(Azure A) obtained from the buffer saturated with chloroform. Sensors 2021, 21, 2949. [Google Scholar] [CrossRef]
- Stoikov, D.I.; Porfir’eva, A.V.; Shurpik, D.N.; Stoikov, I.I.; Evtyugin, G.A. Electrochemical DNA sensors on the basis of electropolymerized thionine and Azure B with addition of pillar[5]arene as an electron transfer mediator. Russ. Chem. Bull. 2019, 68, 431–437. [Google Scholar] [CrossRef]
- Diaz, A.F.; Logan, J.A. Electroactive polyaniline films. J. Electroanal. Chem. 1980, 111, 111–114. [Google Scholar] [CrossRef]
- Diaz, A.F.; Kanazawa, K.K.; Gardini, G.P. Electrochemical polymerization of pyrrole. J. Chem. Soc. Chem. Commun. 1979, 635–636. [Google Scholar] [CrossRef]
- Barsan, M.M.; Pinto, E.M.; Brett, C.M.A. Electrosynthesis and electrochemical characterisation of phenazine polymers for application in biosensors. Electrochim. Acta 2008, 53, 3973–3982. [Google Scholar] [CrossRef]
- Topçu, E.; Alanyalıoğlu, M. Electrochemical formation of poly(thionine) thin films: The effect of amine group on the polymeric film formation of phenothiazine dyes. J. Appl. Polym. Sci. 2014, 131, 39686. [Google Scholar] [CrossRef]
- Gligor, D.; Dilgin, Y.; Popescu, I.C.; Gorton, L. Poly-phenothiazine derivative-modified glassy carbon electrode for NADH electrocatalytic oxidation. Electrochim. Acta 2009, 54, 3124–3128. [Google Scholar] [CrossRef]
- Puskás, Z.; Inzelt, G. Formation and redox transformations of polyphenazine. Electrochim. Acta 2005, 50, 1481–1490. [Google Scholar] [CrossRef]
- Karyakin, A.A. Chapter 5. Electropolymerized azines: A new group of electroactive polymers. In Electropolymerization: Concepts, Materials and Applications; Cosnier, S., Karyakin, A., Eds.; Willey VCH: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Seifi, A.; Afkhami, A.; Madrakian, T. Highly sensitive and simultaneous electrochemical determination of lead and cadmium ions by poly(thionine)/MWCNTs-modified glassy carbon electrode in the presence of bismuth ions. J. Appl. Electrochem. 2022, 52, 1513–1523. [Google Scholar] [CrossRef]
- Prado, N.S.; Silva, L.A.J.; Takeuchi, R.M.; Richter, E.M.; Santos, A.L.D.; Falcão, E.H.L. Graphite sheets modified with poly(methylene blue) films: A cost-effective approach for the electrochemical sensing of the antibiotic nitrofurantoin. Microchem. J. 2022, 177, 107289. [Google Scholar] [CrossRef]
- Soranzo, T.; Ben Tahar, A.; Chmayssem, A.; Zelsmann, M.; Vadgama, P.; Lenormand, J.-L.; Cinquin, P.; Martin, D.K.; Zebda, A. Electrochemical biosensing of glucose based on the enzymatic reduction of glucose. Sensors 2022, 22, 7105. [Google Scholar] [CrossRef]
- Jiménez-Fiérrez, F.; González-Sánchez, M.I.; Jiménez-Pérez, R.; Iniesta, J.; Valero, E. Glucose biosensor based on disposable activated carbon electrodes modified with platinum nanoparticles electrodeposited on poly(Azure A). Sensors 2020, 20, 4489. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Qi, B.; Yang, L.; Guo, L. Ordered mesoporous carbon functionalized with poly-azure B for electrocatalytic application. J. Electroanal. Chem. 2010, 643, 52–57. [Google Scholar] [CrossRef]
- Lin, K.-C.; Yin, C.-Y.; Chen, S.-M. An electrochemical biosensor for determination of hydrogen peroxide using nanocomposite of poly(methylene blue) and FAD hybrid film. Sens. Actuators B 2011, 157, 202–210. [Google Scholar] [CrossRef]
- Ding, M.; Hou, T.; Niu, H.; Zhang, N.; Guan, P.; Hu, X. Electrocatalytic oxidation of NADH at graphene-modified electrodes based on electropolymerized poly(thionine-methylene blue) films from nature deep eutectic solvents. J. Electroanal. Chem. 2022, 920, 116602. [Google Scholar] [CrossRef]
- Tiravia, M.; Sabuzi, F.; Cirulli, M.; Pezzola, S.; Di Carmine, G.; Cicero, D.O.; Floris, B.; Conte, V.; Galloni, P. 3,7-Bis(N-methyl-N-phenylamino)phenothiazinium salt: Improved synthesis and aggregation behavior in solution. Eur. J. Org. Chem. 2019, 2019, 3208–3216. [Google Scholar] [CrossRef]
- Peterson, B.M.; Shen, L.; Lopez, G.J.; Gannett, C.N.; Ren, D.; Abruña, H.D.; Fors, B.P. Elucidation of the electrochemical behavior of phenothiazine-based polyaromatic amines. Tetrahedron 2019, 75, 4244–4249. [Google Scholar] [CrossRef]
- Padnya, P.L.; Khadieva, A.I.; Stoikov, I.I. Current achievements and perspectives in synthesis and applications of 3,7-disubstituted phenothiazines as methylene blue analogues. Dyes Pigment. 2022, 208, 110806. [Google Scholar] [CrossRef]
- Khadieva, A.; Rayanov, M.; Shibaeva, K.; Piskunov, A.; Padnya, P.; Stoikov, I. Towards asymmetrical methylene blue analogues: Synthesis and reactivity of 3-N′-arylaminophenothiazines. Molecules 2022, 27, 3024. [Google Scholar] [CrossRef]
- Wainwright, M.; McLean, A. Rational design of phenothiazinium derivatives and photoantimicrobial drug discovery. Dyes Pigment. 2017, 136, 590–600. [Google Scholar] [CrossRef]
- Kuzin, Y.I.; Padnya, P.L.; Stoikov, I.I.; Gorbatchuk, V.V.; Stoikov, D.I.; Khadieva, A.I.; Evtugyn, G.A. Electrochemical behavior of the monomeric and polymeric forms of N-phenyl-3-(phenylimino)-3H-phenothiazin-7-amine. Electrochim. Acta 2020, 345, 136195. [Google Scholar] [CrossRef]
- Salehan, P.; Ensafi, A.A.; Mousaabadi, K.Z.; Ghasemi, J.B.; Aghaee, E.; Rezaei, B. A theoretical and experimental study of polyaniline/GCE and DNA G-quadruplex conformation as an impedimetric biosensor for the determination of potassium ions. Chemosphere 2022, 292, 133460. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Deshmukh, M.A.; Apetrei, R.-M.; Ramanaviciene, A.; Plikusiene, I.; Morkvenaite-Vilkonciene, I.; Thorat, H.N.; Shirsat, M.D.; Ramanavicius, A. Chapter 15—Conducting polymers—Versatile tools in analytical systems for the determination of biomarkers and biologically active compounds. The detection of biomarkers: Past, present, and the future prospects. In The Detection of Biomarkers. Past, Present and the Future Prospects; Academic Press: Cambridge, MA, USA, 2022; pp. 407–434. [Google Scholar] [CrossRef]
- Tran, L.T.; Tran, H.V.; Cao, H.H.; Tran, T.H.; Huynh, C.D. Electrochemically effective surface area of a polyaniline nanowire-based platinum microelectrode and development of an electrochemical DNA sensor. J. Nanotechnol. 2022, 2022, 8947080. [Google Scholar] [CrossRef]
- Khadieva, A.; Gorbachuk, V.; Shurpik, D.; Stoikov, I. Synthesis of tris-pillar[5]arene and its association with phenothiazine dye: Colorimetric recognition of anions. Molecules 2019, 24, 1807. [Google Scholar] [CrossRef] [PubMed]
- Evtugyn, G.; Porfireva, A.; Hianik, T. Electropolymerized materials for biosensors. In Advanced Bioelectronics Materials; Tiwari, A., Patra, H.K., Turner, A.P.F., Eds.; Wiley: New York, NY, USA, 2015; pp. 89–185. [Google Scholar] [CrossRef]
- Kuzin, Y.I.; Khadieva, A.I.; Padnya, P.L.; Khannanov, A.A.; Kutyreva, M.P.; Stoikov, I.I.; Evtugyn, G.A. Electrochemistry of new derivatives of phenothiazine: Electrode kinetics and electropolymerization conditions. Electrochim. Acta 2021, 375, 137985. [Google Scholar] [CrossRef]
- Suprun, E.V.; Kutdusova, G.R.; Khmeleva, S.A.; Radko, S.P. Towards deeper understanding of DNA electrochemical oxidation on carbon electrodes. Electrochem. Commun. 2021, 124, 106947. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical impedance spectroscopy in the characterisation and application of modified electrodes for electrochemical sensors and biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Kuzin, Y.; Kappo, D.; Porfireva, A.; Shurpik, D.; Stoikov, I.; Evtugyn, G.; Hianik, T. Electrochemical DNA sensor based on carbon black—Poly(neutral red) composite for detection of oxidative DNA damage. Sensors 2018, 18, 3489. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Malanina, A.N.; Kuzin, Y.I.; Ivanov, A.N.; Ziyatdinova, G.K.; Shurpik, D.N.; Stoikov, I.I.; Evtugyn, G.A. Polyelectrolyte polyethylenimine-DNA complexes in the composition of voltammetric sensors for detecting DNA damage. J. Anal. Chem. 2022, 77, 185–194. [Google Scholar] [CrossRef]
- Gunvén, G.; Theve, N.O.; Peterson, C. Serum and tissue concentrations of doxorubicin after IV administration of doxorubicin or doxorubicin-DNA complex to patients with gastrointestinal cancer. Cancer Chemother. Pharmacol. 1986, 17, 153–156. [Google Scholar] [CrossRef]
- Chai, M.; Wientjes, M.G.; Badalament, R.A.; Burgers, J.K.; Au, J.L.-S. Pharmacokinetics of intra vesical doxorubicin in superficial bladder cancer patients. J. Urol. 1994, 152, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Krarup-Hansen, A.; Wassermann, K.; Rasmussen, S.N.; Dalmark, M. Pharmacokinetics of doxorubicin in man with induced acid or alkaline urine. Acta Oncol. 1988, 27, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Maudens, K.E.; Stove, C.P.; Lambert, W.E. Quantitative liquid chromatographic analysis of anthracyclines in biological fluids. J. Chromatogr. B 2011, 879, 2471–2486. [Google Scholar] [CrossRef] [PubMed]
- Shamagsumova, R.; Porfireva, A.; Stepanova, V.; Osin, Y.; Evtugyn, G.; Hianik, T. Polyaniline–DNA based sensor for the detection of anthracycline drugs. Sens. Actuators B 2015, 220, 573–582. [Google Scholar] [CrossRef]
- Hajian, R.; Mehrayin, Z.; Mohagheghian, M.; Zafari, M.; Hosseini, P.; Shams, N. Fabrication of an electrochemical sensor based on carbon nanotubes modified with gold nanoparticles for determination of valrubicin as a chemotherapy drug: Valrubicin-DNA interaction. Mater. Sci. Eng. C 2015, 49, 769–775. [Google Scholar] [CrossRef]
- Hongpaisan, J.; Roomans, G.M. Retaining ionic concentrations during in vitro storage of tissue for microanalytical studies. J. Microsc. 1999, 193, 257–267. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.R.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.; Zhou, M.; Guo, L. A nanocomposite prepared from metal-free mesoporous carbon nanospheres and graphene oxide for voltammetric determination of doxorubicin. Microchim. Acta 2019, 186, 639. [Google Scholar] [CrossRef]
- Vacek, J.; Havran, L.; Fojta, M. Ex situ voltammetry and chronopotentiometry of doxorubicin at a pyrolytic graphite elec-trode: Redox and catalytic properties and analytical applications. Electroanalysis 2009, 21, 21399–22144. [Google Scholar] [CrossRef]
- Skalová, Š.; Langmaier, J.; Barek, J.; Vyskočil, V.; Navrátils, T. Doxorubicin determination using two novel voltammetric approaches: A comparative study. Electrochim. Acta 2020, 330, 135180. [Google Scholar] [CrossRef]
- Ali, A.-M.B.H.; Rageh, A.H.; Abdel-aal, F.A.M.; Mohamed, A.-M.I. Anatase titanium oxide nanoparticles and multi-walled carbon nanotubes-modified carbon paste electrode for simultaneous determination of avanafil and doxorubicin in plasma samples. Microchem. J. 2023, 185, 108261. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, V.; Thakur, N.; Tejwan, N.; Sharma, A.; Das, J. Selective and sensitive electrochemical detection of doxorubicin via a novel magnesium oxide/carbon dot nanocomposite based sensor. Inorg. Chem. Commun. 2023, 150, 110527. [Google Scholar] [CrossRef]
- Abbasi, M.; Ezazi, M.; Jouyban, A.; Lulek, E.; Asadpour-Zeynali, K.; Ertas, Y.N.; Houshyar, J.; Mokhtarzadeh, A.; Soleymani, J. An ultrasensitive and preprocessing-free electrochemical platform for the detection of doxorubicin based on tryptophan/polyethylene glycol-cobalt ferrite nanoparticles modified electrodes. Microchem. J. 2022, 183, 108055. [Google Scholar] [CrossRef]
- Porfireva, A.; Vorobev, V.; Babkina, S.; Evtugyn, G. Electrochemical sensor based on poly(Azure B)-DNA composite for doxorubicin determination. Sensors 2019, 19, 2085. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, T.; Porfireva, A.; Evtugyn, G.; Hianik, T. Electrochemical DNA sensors with layered polyaniline-DNA coating for detection of specific DNA interactions. Sensors 2019, 19, 469. [Google Scholar] [CrossRef]
- Peng, A.; Xu, H.; Luo, C.; Ding, H. Application of a disposable doxorubicin sensor for direct determination of clinical drug concentration in patient blood. Int. J. Electrochem. Sci. 2016, 11, 6266–6278. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Budnikov, H. Electrochemical biosensors based on native DNA and nanosized mediator for the detection of anthracycline preparations. Electroanalysis 2015, 27, 629–637. [Google Scholar] [CrossRef]
- Kulikova, T.; Porfireva, A.; Rogov, A.; Evtugyn, G. Electrochemical DNA sensor based on acridine yellow adsorbed on glassy carbon electrode. Sensors 2021, 21, 7763. [Google Scholar] [CrossRef]
- Kappo, D.; Shurpik, D.; Padnya, P.; Stoikov, I.; Rogov, A.; Evtugyn, G. Electrochemical DNA sensor based on carbon black-poly(methylene blue)-poly(neutral red) composite. Biosensors 2022, 12, 329. [Google Scholar] [CrossRef]
- Karadurmus, L.; Dogan-Topal, B.; Kurbanoglu, S.; Shah, A.; Ozkan, S.A. The interaction between DNA and three intercalating anthracyclines using electrochemical DNA nanobiosensor based on metal nanoparticles modified screen-printed electrode. Micromachines 2021, 12, 1337. [Google Scholar] [CrossRef]
- Moghadam, F.H.; Taher, M.A.; Karimi-Maleh, H. Doxorubicin anticancer drug monitoring by ds-DNA-based electrochemical biosensor in clinical samples. Micromachines 2021, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Yamamoto, T.; Nagashima, S.; Ogata, G.; Hibino, H.; Einaga, Y. An electrochemical aptamer-based sensor prepared by utilizing the strong interaction between a DNA aptamer and diamond. Analyst 2020, 145, 544–549. [Google Scholar] [CrossRef] [PubMed]












| Modifier | RS, Ω | Rct, kΩ | Q, µF | N |
|---|---|---|---|---|
| PolyPhTz | 162 ± 8 | 24.5 ± 3 | 0.27 ± 0.04 | 0.86 |
| PolyPhTz/DNA1 | 191 ± 8 | 51.2 ± 4 | 0.32 ± 0.05 | 0.91 |
| PolyPhTz/DNA2 | 171 ± 10 | 134 ± 6 | 0.08 ± 0.03 | 0.88 |
| PolyPhTz/DNA3 | 172 ± 12 | 277 ± 6 | 0.04 ± 0.01 | 0.90 |
| DNA Source | a ± Δa | b ± Δb | R2 | n | Linear Concentration Range | LOD |
|---|---|---|---|---|---|---|
| DNA1 | 0.99 ± 0.02 | 0.070 ±0.003 | 0.9898 | 7 | 10 pM–0.2 mM | 5 pM |
| DNA2 | 1.12 ± 0.11 | 0.078 ± 0.012 | 0.9784 | 5 | 20 pM–12 nM | 10 pM |
| DNA3 | 1.06 ± 0.06 | 0.089 ±0.009 | 0.9529 | 5 | 15 nM–0.3 mM | 50 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malanina, A.; Kuzin, Y.; Khadieva, A.; Shibaeva, K.; Padnya, P.; Stoikov, I.; Evtugyn, G. Voltammetric Sensor for Doxorubicin Determination Based on Self-Assembled DNA-Polyphenothiazine Composite. Nanomaterials 2023, 13, 2369. https://doi.org/10.3390/nano13162369
Malanina A, Kuzin Y, Khadieva A, Shibaeva K, Padnya P, Stoikov I, Evtugyn G. Voltammetric Sensor for Doxorubicin Determination Based on Self-Assembled DNA-Polyphenothiazine Composite. Nanomaterials. 2023; 13(16):2369. https://doi.org/10.3390/nano13162369
Chicago/Turabian StyleMalanina, Anastasiya, Yurii Kuzin, Alena Khadieva, Kseniya Shibaeva, Pavel Padnya, Ivan Stoikov, and Gennady Evtugyn. 2023. "Voltammetric Sensor for Doxorubicin Determination Based on Self-Assembled DNA-Polyphenothiazine Composite" Nanomaterials 13, no. 16: 2369. https://doi.org/10.3390/nano13162369