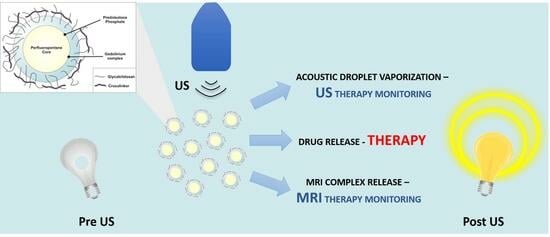
Hard-Shelled Glycol Chitosan Nanoparticles for Dual MRI/US Detection of Drug Delivery/Release: A Proof-of-Concept Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of PLP’s Partition Coefficient and Critical Micellar Concentration
2.3. Preparation of the Theranostic Glycol Chitosan Nanobubbles
2.4. Physical Characterization and Stability Assessment of the Nanobubbles
2.5. Determination of the Hemolytic Activity
2.6. Quantitative Determination of Loaded PLP
2.7. In Vitro Release Experiments
2.8. Cell Viability and Cytotoxicity Assay
2.9. Cellular Uptake Experiments
2.10. Relaxometric Measurements
2.11. Ultrasound Imaging
2.12. Statistical Analysis
3. Results
3.1. General NB Characterization
3.2. In Vitro Release Kinetics of PLP and Gd-DTPAMA-CHOL from Nanobubbles
3.3. Relaxometric Characterization
3.4. Cell Uptake and Viability
3.5. US imaging Detection of the Theranostic Nanobubbles
3.6. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartlett, G.; Antoun, J.; Zgheib, N.K. Theranostics in primary care: Pharmacogenomics tests and beyond. Expert Rev. Mol. Diagn. 2012, 12, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Terreno, E.; Uggeri, F.; Aime, S. Image guided therapy: The advent of theranostic agents. J. Control. Release 2012, 161, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive Imaging of Nanomedicines and Nanotheranostics: Principles, Progress, and Prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef]
- Kunjachan, S.; Jayapaul, J.; Mertens, M.E.; Storm, G.; Kiessling, F.; Lammers, T. Theranostic systems and strategies for monitoring nanomedicine-mediated drug targeting. Curr. Pharm. Biotechnol. 2012, 13, 609–622. [Google Scholar] [CrossRef]
- Feshitan, J.A.; Vlachos, F.; Sirsi, S.R.; Konofagou, E.E.; Borden, M.A. Theranostic Gd(III)-lipid microbubbles for MRI-guided focused ultrasound surgery. Biomaterials 2012, 33, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Liao, A.H.; Liu, H.L.; Su, C.H.; Hua, M.Y.; Yang, H.W.; Weng, Y.T.; Hsu, P.H.; Huang, S.M.; Wu, S.Y.; Wang, H.E.; et al. Paramagnetic perfluorocarbon-filled albumin-(Gd-DTPA) microbubbles for the induction of focused-ultrasound-induced blood-brain barrier opening and concurrent MR and ultrasound imaging. Phys. Med. Biol. 2012, 57, 2787–2802. [Google Scholar] [CrossRef]
- Sciallero, C.; Balbi, L.; Paradossi, G.; Trucco, A. Magnetic resonance and ultrasound contrast imaging of polymer-shelled microbubbles loaded with iron oxide nanoparticles. R. Soc. Open Sci. 2016, 3, 160063. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Lin, X.; Wu, M.; Zhao, B.; Lin, R.; Zhang, D.; Zhang, Y.; Liu, G.; Liu, X.; Liu, J. Core-shell NaGdF4@CaCO3 nanoparticles for enhanced magnetic resonance/ultrasonic dual-modal imaging via tumor acidic micro-enviroment triggering. Sci. Rep. 2017, 7, 5370. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.I.; Ha, S.W.; Lee, H.J. An ultrasound-responsive dual-modal US/T1-MRI contrast agent for potential diagnosis of prostate cancer. J. Magn. Reson. Imaging 2018, 48, 1610–1616. [Google Scholar] [CrossRef]
- Waqar, H.; Riaz, R.; Ahmed, N.M.; Majeed, A.I.; Abbas, S.R. Monodisperse magnetic lecithin-PFP submicron bubbles as dual imaging contrast agents for ultrasound (US) and MRI. RSC Adv. 2022, 12, 10504–10513. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Argenziano, M.; Grillo, G.; Ferrara, B.; Pizzimenti, S.; Barrera, G.; Cravotto, G.; Guiot, C.; Stura, I.; Cavalli, R.; et al. Low-dose curcuminoid-loaded in dextran nanobubbles can prevent metastatic spreading in prostate cancer cells. Nanotechnology 2019, 30, 214004. [Google Scholar] [CrossRef] [PubMed]
- Güvener, N.; Appold, L.; de Lorenzi, F.; Golombek, S.K.; Rizzo, L.Y.; Lammers, T.; Kiessling, F. Recent advances in ultrasound-based diagnosis and therapy with micro- and nanometer-sized formulations. Methods 2017, 130, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Zullino, S.; Argenziano, M.; Stura, I.; Guiot, C.; Cavalli, R. From Micro- to Nano-Multifunctional Theranostic Platform: Effective Ultrasound Imaging Is Not Just a Matter of Scale. Mol. Imaging 2018, 17, 1536012118778216. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Abenojar, E.C.; Perera, R.; De Leon, A.C.; An, T.; Exner, A.A. Time-intensity-curve Analysis and Tumor Extravasation of Nanobubble Ultrasound Contrast Agents. Ultrasound Med. Biol. 2019, 45, 2502–2514. [Google Scholar] [CrossRef]
- Capolla, S.; Argenziano, M.; Bozzer, S.; D’Agaro, T.; Bittolo, T.; De Leo, L.; Not, T.; Busato, D.; Dal Bo, M.; Toffoli, G.; et al. Targeted chitosan nanobubbles as a strategy to down-regulate microRNA-17 into B-cell lymphoma models. Front. Immunol. 2023, 14, 1200310. [Google Scholar] [CrossRef]
- Macor, P.; Durigutto, P.; Argenziano, M.; Smith-Jackson, K.; Capolla, S.; Di Leonardo, V.; Marchbank, K.; Tolva, V.S.; Semeraro, F.; Ammollo, C.T.; et al. Plasminogen activator-coated nanobubbles targeting cell-bound β2-glycoprotein I as a novel thrombus-specific thrombolytic strategy. Haematologica 2022, 108, 1861. [Google Scholar] [CrossRef]
- Zullino, S.; Argenziano, M.; Ansari, S.; Ciprian, R.; Nasi, L.; Albertini, F.; Cavalli, R.; Guiot, C. Superparamagnetic Oxygen-Loaded Nanobubbles to Enhance Tumor Oxygenation During Hyperthermia. Front. Pharmacol. 2019, 10, 1001. [Google Scholar] [CrossRef]
- Ficiarà, E.; Ansari, S.A.; Argenziano, M.; Cangemi, L.; Monge, C.; Cavalli, R.; D’Agata, F. Beyond Oncological Hyperthermia: Physically Drivable Magnetic Nanobubbles as Novel Multipurpose Theranostic Carriers in the Central Nervous System. Molecules 2020, 25, 2104. [Google Scholar] [CrossRef]
- Luo, B.; Zhang, H.; Liu, X.; Rao, R.; Wu, Y.; Liu, W. Novel DiR and SPIO nanoparticles embedded PEG-PLGA nanobubbles as a multimodalimaging contrast agent. Biomed. Mater. Eng. 2015, 26, S911–S916. [Google Scholar] [CrossRef]
- Cavalli, R.; Argenziano, M.; Vigna, E.; Giustetto, P.; Torres, E.; Aime, S.; Terreno, E. Preparation and in vitro characterization of chitosan nanobubbles as theranostic agents. Colloids Surf. B Biointerfaces 2015, 129, 39–46. [Google Scholar] [CrossRef]
- Huang, H.Y.; Liu, H.L.; Hsu, P.H.; Chiang, C.S.; Tsai, C.H.; Chi, H.S.; Chen, S.Y.; Chen, Y.Y. A multitheragnostic nanobubble system to induce blood-brain barrier disruption with magnetically guided focused ultrasound. Adv. Mater. 2015, 27, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, Y.; Liu, W.; Guan, W.; Liu, H.; Duan, Y.; Chen, Y. Magnetic polymeric nanobubbles with optimized core size for MRI/ultrasound bimodal molecular imaging of prostate cancer. Nanomedicine 2020, 5, 2901–2916. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Luo, Y.; Zhao, Y.; Liu, X.; Zhao, J.; Luo, J.; Zhang, Q.; Ran, H.; Wang, Z.; Guo, D. Magnetic nanobubbles with potential for targeted drug delivery and trimodal imaging in breast cancer: An in vitro study. Nanomedicine 2017, 12, 991–1009. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Cheng, X.; Li, J. Lipid nanobubbles as an ultrasound-triggered artesunate delivery system for imaging-guided, tumor-targeted chemotherapy. Onco Targets Ther. 2019, 12, 1841–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Guo, L.; Shi, D.; Duan, S.; Li, J. Biocompatible Chitosan Nanobubbles for Ultrasound-Mediated Targeted Delivery of Doxorubicin. Nanoscale Res. Lett. 2019, 14, 24. [Google Scholar] [CrossRef]
- Jin, Z.; Chang, J.; Dou, P.; Jin, S.; Jiao, M.; Tang, H.; Jiang, W.; Ren, W.; Zheng, S. Tumor Targeted Multifunctional Magnetic Nanobubbles for MR/US Dual Imaging and Focused Ultrasound Triggered Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 586874. [Google Scholar] [CrossRef]
- Argenziano, M.; Bessone, F.; Dianzani, C.; Cucci, M.A.; Grattarola, M.; Pizzimenti, S.; Cavalli, R. Ultrasound-Responsive Nrf2-Targeting siRNA-Loaded Nanobubbles for Enhancing the Treatment of Melanoma. Pharmaceutics 2022, 14, 341. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, G.; Li, M.; Ma, L.; Huang, J.; Qiu, L. Ultrasound-Augmented Phase Transition Nanobubbles for Targeted Treatment of Paclitaxel-Resistant Cancer. Bioconjug. Chem. 2020, 31, 2008–2020. [Google Scholar] [CrossRef]
- Argenziano, M.; Banche, G.; Luganini, A.; Finesso, N.; Allizond, V.; Gulino, G.R.; Khadjavi, A.; Spagnolo, R.; Tullio, V.; Giribaldi, G.; et al. Vancomycin-loaded nanobubbles: A new platform for controlled antibiotic delivery against methicillin-resistant Staphylococcus aureus infections. Int. J. Pharm. 2017, 523, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Chen, Y.; Yu, T.; Guo, Y.; Liu, F.; Yao, Y.; Li, P.; Wang, D.; Wang, Z.; Chen, Y.; et al. Drug Release from Phase-Changeable Nanodroplets Triggered by Low-Intensity Focused Ultrasound. Theranostics 2018, 8, 1327–1339. [Google Scholar] [CrossRef]
- Anelli, P.L.; Lattuada, L.; Lorusso, V.; Lux, G.; Morisetti, A.; Morosini, P.; Serleti, M.; Uggeri, F. Conjugates of gadolinium complexes to bile acids as hepatocyte-directed contrast agents for magnetic resonance imaging. J. Med. Chem. 2004, 47, 3629–3641. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Botta, M.; Terreno, E. Gd(III)-Based Contrast Agents for MRI. Adv. Inorg. Chem. 2006, 57, 173–237. [Google Scholar]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Parac Vogt, T.N.; Kimpe, K.; Laurent, S.; Piérart, C.; Vander Elst, L.; Muller, R.N.; Binnemans, K. Gadolinium DTPA-Monoamide Complexes Incorporated into Mixed Micelles as Possible MRI Contrast Agents. Eur. J. Inorg. Chem. 2004, 2004, 3538–3543. [Google Scholar] [CrossRef]
- Filippi, M.; Martinelli, J.; Mulas, G.; Ferraretto, M.; Teirlinck, E.; Botta, M.; Tei, L.; Terreno, E. Dendrimersomes: A new vesicular nano-platform for MR-molecular imaging applications. Chem. Commun. 2014, 50, 3453. [Google Scholar] [CrossRef]
- Fringuello Mingo, A.; Colombo Serra, S.; Baroni, S.; Cabella, C.; Napolitano, R.; Hawala, I.; Carnovale, I.M.; Lattuada, L.; Tedoldi, F.; Aime, S. Macrocyclic paramagnetic agents for MRI: Determinants of relaxivity and strategies for their improvement. Magn. Reson. Med. 2017, 78, 1523–1532. [Google Scholar] [CrossRef]
- Cittadino, E.; Ferraretto, M.; Torres, E.; Maiocchi, A.; Crielaard, B.J.; Lammers, T.; Storm, G.; Aime, S.; Terreno, E. MRI evaluation of the antitumor activity of paramagnetic liposomes loaded with prednisolone phosphate. Eur. J. Pharma. Sci. 2012, 45, 436–441. [Google Scholar] [CrossRef]
- Kripfgans, O.D.; Fowlkes, J.B.; Miller, D.L.; Eldevik, O.P.; Carson, P.L. Acoustic Droplet Vaporization for Therapeutic and Diagnostic Applications. Ultrasound Med. Biol. 2000, 26, 1177–1189. [Google Scholar] [CrossRef]
- Loskutova, K.; Grishenkov, D.; Ghorbani, M. Review on Acoustic Droplet Vaporization in Ultrasound Diagnostics and Therapeutics. BioMed Res. Int. 2019, 2019, 9480193. [Google Scholar] [CrossRef]
- de Leon, A.; Perera, R.; Nittayacharn, P.; Cooley, M.; Jung, O.; Exner, A.A. Ultrasound Contrast Agents and Delivery Systems in Cancer Detection and Therapy. Adv. Cancer Res. 2018, 139, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Ren, X.; Nie, F.; Li, T.; Lv, W.; Li, H.; Zhang, Y. Current advances in ultrasound-combined nanobubbles for cancer-targeted therapy: A review of the current status and future perspectives. RSC Adv. 2021, 11, 12915–12928. [Google Scholar] [CrossRef] [PubMed]
- Lea-Banks, H.; O’Reilly, M.A.; Hynynen, K. Ultrasound-responsive droplets for therapy: A review. J. Control. Release 2019, 293, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Kee, A.L.Y.; Teo, B.M. Biomedical applications of acoustically responsive phase shift nanodroplets: Current status and future directions. Ultrason. Sonochem. 2019, 56, 37–45. [Google Scholar] [CrossRef]
- Spatarelu, C.P.; Jandhyala, S.; Luke, G.P. Dual-drug loaded ultrasound-responsive nanodroplets for on-demand combination chemotherapy. Ultrasonics 2023, 133, 107056. [Google Scholar] [CrossRef]
- Cavalli, R.; Soster, M.; Argenziano, M. Nanobubbles: A promising efficient tool for therapeutic delivery. Ther. Deliv. 2016, 7, 117–138. [Google Scholar] [CrossRef]
- Wiegand, C.; Winter, D.; Hipler, U.C. Molecular-weight-dependent toxic effects of chitosans on the human keratinocyte cell line HaCaT. Skin Pharmacol. Physiol. 2010, 23, 164–170. [Google Scholar] [CrossRef]
- Argenziano, M.; Bressan, B.; Luganini, A.; Finesso, N.; Genova, T.; Troia, A.; Giribaldi, G.; Banche, G.; Mandras, N.; Cuffini, A.M.; et al. Comparative Evaluation of Different Chitosan Species and Derivatives as Candidate Biomaterials for Oxygen-Loaded Nanodroplet Formulations to Treat Chronic Wounds. Mar. Drugs 2021, 19, 112. [Google Scholar] [CrossRef]
- Argenziano, M.; Occhipinti, S.; Scomparin, A.; Angelini, C.; Novelli, F.; Soster, M.; Giovarelli, M.; Cavalli, R. Exploring chitosan-shelled nanobubbles to improve HER2+ immunotherapy via dendritic cell targeting. Drug Deliv. Transl. Res. 2022, 12, 2007–2018. [Google Scholar] [CrossRef]
- Marano, F.; Argenziano, M.; Frairia, R.; Adamini, A.; Bosco, O.; Rinella, L.; Fortunati, N.; Cavalli, R.; Catalano, M.G. Doxorubicin-Loaded Nanobubbles Combined with Extracorporeal Shock Waves: Basis for a New Drug Delivery Tool in Anaplastic Thyroid Cancer. Thyroid 2016, 26, 705–716. [Google Scholar] [CrossRef]
- Marano, F.; Frairia, R.; Rinella, L.; Argenziano, M.; Bussolati, B.; Grange, C.; Mastrocola, R.; Castellano, I.; Berta, L.; Cavalli, R.; et al. Combining doxorubicin-nanobubbles and shockwaves for anaplastic thyroid cancer treatment: Preclinical study in a xenograft mouse model. Endocr. Relat. Cancer 2017, 24, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Marano, F.; Rinella, L.; Argenziano, M.; Cavalli, R.; Sassi, F.; D’Amelio, P.; Battaglia, A.; Gontero, P.; Bosco, O.; Peluso, R.; et al. Targeting Taxanes to Castration-Resistant Prostate Cancer Cells by Nanobubbles and Extracorporeal Shock Waves. PLoS ONE 2016, 11, e0168553. [Google Scholar] [CrossRef] [Green Version]
- Yhee, J.Y.; Son, S.; Kim, S.H.; Park, K.; Choi, K.; Kwon, I.C. Self-assembled glycol chitosan nanoparticles for disease-specific theranostics. J. Control. Release 2014, 193, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jiang, Y.; Lin, M.; Zhang, J.; Guo, H.; Yang, F.; Leung, W.; Xu, C. Ultrasound-Responsive Materials for Drug/Gene Delivery. Front. Pharmacol. 2020, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor microenvironment targeted nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Bilensoy, E. Cationic nanoparticles for cancer therapy. Expert Opin. Drug Deliv. 2010, 7, 795–809. [Google Scholar] [CrossRef]









| Hard-Shelled Nanodroplets Formulation | Average Diameter ± SD (nm) | Polydispersity Index | Z-Potential ± SD (mV) |
|---|---|---|---|
| Blank nanodroplets | 538.7 ± 24.7 | 0.13 | +17.6 ± 2.9 |
| Gd-DTPAMA-Chol/PLP | 540.2 ± 30.5 | 0.11 | +14.3 ± 2.5 |
| Gd-DTPAMA-Chol/PLP/coumarin 6 | 562.6 ± 41.4 | 0.11 | +14.1 ± 1.4 |
| Gd-DTPAMA-Chol/PLP/rhodamine–DSPE | 553.3 ± 38.2 | 0.11 | +13.4 ± 1.2 |
| Average Diameter ± SD (nm) | Polydispersity Index | Z-Potential ± SD (mV) | |
|---|---|---|---|
| Time 0 | 545.6 ± 18.4 | 0.110 | +12.9 ± 3.2 |
| 24 h | 550.1 ± 19.5 | 0.112 | +13.4 ± 4.0 |
| 96 h | 551.3 ± 13.6 | 0.111 | +13.2 ± 3.1 |
| 15 days | 547.6 ± 15.2 | 0.112 | +12.8 ± 4.3 |
| 30 days | 550.8 ± 12.5 | 0.108 | +13.0 ± 3.5 |
| 90 days | 548.4 ± 17.3 | 0.109 | +13.5 ± 2.5 |
| Average Diameter ± SD (nm) | Polydispersity Index | Z-Potential ± SD (mV) | |
|---|---|---|---|
| No US | 534.3 ± 12.6 | 0.111 | +13.1 ± 4.5 |
| Short US application | 1088 ± 22.8 | 0.125 | +13.3 ± 2.4 |
| Δ2 × 1019 (s−2) | 310tV (ps) | 310tR (ns) | 310tM (ns) | |
|---|---|---|---|---|
| Gd-DTPAMA-CHOL | 4.8 | 24.5 | 0.11 | 300–500 |
| Theranostic nanobubbles | 1.2 | 11.1 | 3.95 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroni, S.; Argenziano, M.; La Cava, F.; Soster, M.; Garello, F.; Lembo, D.; Cavalli, R.; Terreno, E. Hard-Shelled Glycol Chitosan Nanoparticles for Dual MRI/US Detection of Drug Delivery/Release: A Proof-of-Concept Study. Nanomaterials 2023, 13, 2227. https://doi.org/10.3390/nano13152227
Baroni S, Argenziano M, La Cava F, Soster M, Garello F, Lembo D, Cavalli R, Terreno E. Hard-Shelled Glycol Chitosan Nanoparticles for Dual MRI/US Detection of Drug Delivery/Release: A Proof-of-Concept Study. Nanomaterials. 2023; 13(15):2227. https://doi.org/10.3390/nano13152227
Chicago/Turabian StyleBaroni, Simona, Monica Argenziano, Francesca La Cava, Marco Soster, Francesca Garello, David Lembo, Roberta Cavalli, and Enzo Terreno. 2023. "Hard-Shelled Glycol Chitosan Nanoparticles for Dual MRI/US Detection of Drug Delivery/Release: A Proof-of-Concept Study" Nanomaterials 13, no. 15: 2227. https://doi.org/10.3390/nano13152227