Photocatalysts Based on Graphite-like Carbon Nitride with a Low Content of Rhodium and Palladium for Hydrogen Production under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. g-C3N4 Synthesis
2.3. Rh and Pd Deposition
2.4. Photocatalyst Characterization
2.5. Photocatalytic Activity
3. Results
3.1. Photocatalyst Characterization
3.1.1. X-ray Diffraction
3.1.2. UV–Vis Spectroscopy
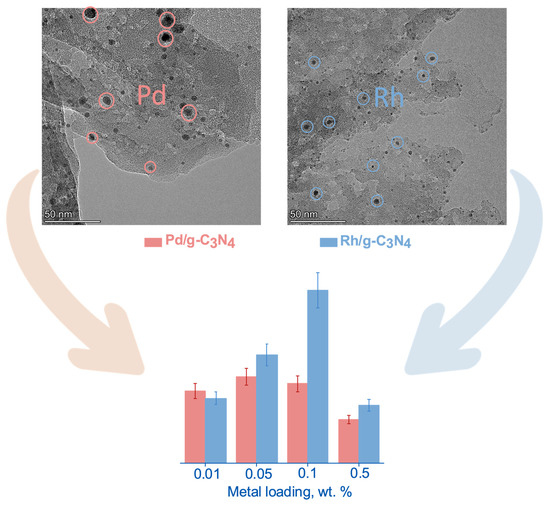
3.1.3. HR TEM Method
3.1.4. XPS Method
3.2. Photocatalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krausmann, F.; Schaffartzik, A.; Mayer, A.; Eisenmenger, N.; Gingrich, S.; Haberl, H.; Fischer-kowalski, M. Social Ecology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- World Meteorological Organization GHG-Bulletin-15_en. Wmo Greenh. Gas Bull. 2019, 15. Available online: https://library.wmo.int/index.php?lvl=notice_display&id=21620 (accessed on 23 July 2023).
- Wang, Z.; Li, C.; Domen, K. Recent Developments in Heterogeneous Photocatalysts for Solar-Driven Overall Water Splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Nagar, R.; Srivastava, S.; Leo, S.; Amaya, S.L.; Tanna, A. Recent Developments in State-of-the-Art Hydrogen Energy Technologies—Review of Hydrogen Storage Materials. Sol. Compass 2023, 5, 100033. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally Friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S.; Buchanan, M.V. The Hydrogen Economy. Phys. Today 2004, 57, 39–44. [Google Scholar] [CrossRef]
- Zou, C.; Li, J.; Zhang, X.; Jin, X.; Xiong, B.; Yu, H.; Liu, X.; Wang, S.; Li, Y.; Zhang, L.; et al. Industrial Status, Technological Progress, Challenges, and Prospects of Hydrogen Energy. Nat. Gas Ind. B 2022, 9, 427–447. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Parmon, V.N. Heterogeneous Semiconductor Photocatalysts for Hydrogen Production from Aqueous Solutions of Electron Donors. Russ. Chem. Rev. 2017, 86, 870–906. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Rajan, A.; Rugma, T.P.; Bernaurdshaw, N. A Review on Particulate Photocatalytic Hydrogen Production System: Progress Made in Achieving High Energy Conversion Efficiency and Key Challenges Ahead. Renew. Sustain. Energy Rev. 2021, 152, 111694. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Şen, Z. Solar Energy in Progress and Future Research Trends. Prog. Energy Combust. Sci. 2004, 30, 367–416. [Google Scholar] [CrossRef]
- Bie, C.; Cheng, B.; Fan, J.; Ho, W.; Yu, J. Enhanced Solar-to-Chemical Energy Conversion of Graphitic Carbon Nitride by Two-Dimensional Cocatalysts. EnergyChem 2021, 3, 100051. [Google Scholar] [CrossRef]
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-Based Heterojunction Photocatalysts. Appl. Surf. Sci. 2018, 430, 53–107. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A Review of Hydrogen Production Processes by Photocatalytic Water Splitting—From Atomistic Catalysis Design to Optimal Reactor Engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Zhurenok, A.V.; Vasilchenko, D.B.; Kozlova, E.A. Comprehensive Review on G-C3N4-Based Photocatalysts for the Photocatalytic Hydrogen Production under Visible Light. Int. J. Mol. Sci. 2023, 24, 346. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Lei, Y.; Wu, X.; Yuan, J.; Chen, J.; Xu, D.; Zhang, X.; Zhang, S.; Liu, P.; Zhang, L.; et al. Tuning the Interfacial Electronic Structure: Via Au Clusters for Boosting Photocatalytic H2 Evolution. J. Mater. Chem. A 2021, 9, 1759–1769. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Weng, B.; Lai, F.; Zhang, M.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A.; Long, J. Site-Sensitive Selective CO2 Photoreduction to CO over Gold Nanoparticles. Angew. Chemie Int. Ed. 2022, 61, e202204563. [Google Scholar] [CrossRef]
- Balraj, G.; Gurrapu, R.; Anil Kumar, A.; Sumalatha, V.; Ayodhya, D. Facile Synthesis and Characterization of Noble Metals Decorated G-C3N4 (g-C3N4/Pt and g-C3N4/Pd) Nanocomposites for Efficient Photocatalytic Production of Schiff Bases. Results Chem. 2022, 4, 100597. [Google Scholar] [CrossRef]
- Krishnan, A.; Yoosuf, M.; Archana, K.; Arsha, A.S.; Viswam, A. Metal Derivative (MD)/g-C3N4 Association in Hydrogen Production: A Study on the Fascinating Chemistry behind, Current Trend & Future Direction. J. Energy Chem. 2023, 80, 562–583. [Google Scholar] [CrossRef]
- Ding, J.; Sun, X.; Wang, Q.; Li, D.S.; Li, X.; Li, X.; Chen, L.; Zhang, X.; Tian, X.; Ostrikov, K. (Ken) Plasma Synthesis of Pt/g-C3N4 Photocatalysts with Enhanced Photocatalytic Hydrogen Generation. J. Alloys Compd. 2021, 873, 159871. [Google Scholar] [CrossRef]
- Trasatti, S. Work Function, Electronegativity, and Electrochemical Behaviour of Metals. III. Electrolytic Hydrogen Evolution in Acid Solutions. J. Electroanal. Chem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Ooka, H.; Huang, J.; Exner, K.S. The Sabatier Principle in Electrocatalysis: Basics, Limitations, and Extensions. Front. Energy Res. 2021, 9, 654460. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.; Nørskov, J.K. Computational High-Throughput Screening of Electrocatalytic Materials for Hydrogen Evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Quaino, P.; Juarez, F.; Santos, E.; Schmickler, W. Volcano Plots in Hydrogen Electrocatalysis-Uses and Abuses. Beilstein J. Nanotechnol. 2014, 5, 846–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ligthart, D.A.J.M.; Quek, X.Y.; Gao, L.; Hensen, E.J.M. Influence of Rh Nanoparticle Size and Composition on the Photocatalytic Water Splitting Performance of Rh/Graphitic Carbon Nitride. Int. J. Hydrogen Energy 2014, 39, 11537–11546. [Google Scholar] [CrossRef]
- Li, F.; Xu, B.; You, X.; Gao, G.; Xu, R.; Wang, X.L.; Yao, Y.F. In-Situ Synthesis of Pd Nanocrystals with Exposed Surface-Active Facets on g-C3N4 for Photocatalytic Hydrogen Generation. Int. J. Hydrogen Energy 2023, 48, 12299–12308. [Google Scholar] [CrossRef]
- Lu, R.; Hu, M.; Xu, C.; Wang, Y.; Zhang, Y.; Xu, B.; Gao, D.; Bi, J.; Fan, G. Hydrogen Evolution from Hydrolysis of Ammonia Borane Catalyzed by Rh/g-C3N4 under Mild Conditions. Int. J. Hydrogen Energy 2018, 43, 7038–7045. [Google Scholar] [CrossRef]
- Peng, Y.; He, Y.; Wang, Y.; Long, Y.; Fan, G. Sustainable One-Pot Construction of Oxygen-Rich Nitrogen-Doped Carbon Nanosheets Stabilized Ultrafine Rh Nanoparticles for Efficient Ammonia Borane Hydrolysis. J. Colloid Interface Sci. 2021, 594, 131–140. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhang, X.L.; Peng, Z.K.; Liu, P.; Zheng, X.C. Highly Efficient Hydrolysis of Ammonia Borane Using Ultrafine Bimetallic RuPd Nanoalloys Encapsulated in Porous G-C3N4. Fuel 2020, 277, 118243. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Jin, C.; Li, Z.; Chai, T.; Zhu, T. Fabrication of Novel Ternary Heterojunctions of Pd/g-C3N4/Bi2MoO6 Hollow Microspheres for Enhanced Visible-Light Photocatalytic Performance toward Organic Pollutant Degradation. Sep. Purif. Technol. 2019, 211, 1–9. [Google Scholar] [CrossRef]
- Yin, Z.; Tian, Y.; Gao, P.; Feng, L.; Liu, Y.; Du, Z.; Zhang, L. Photodegradation Mechanism and Genetic Toxicity of Bezafibrate by Pd/g-C3N4 Catalysts under Simulated Solar Light Irradiation: The Role of Active Species. Chem. Eng. J. 2020, 379, 122294. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Lv, H.; Wang, H.; Zeng, Y.; Yuan, M.; Meng, Q.; Wang, C. Confining Single-Atom Pd on g-C3N4 with Carbon Vacancies towards Enhanced Photocatalytic NO Conversion. Appl. Catal. B Environ. 2021, 284, 119683. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Topchiyan, P.; Tsygankova, A.; Asanova, T.; Kolesov, B.; Bukhtiyarov, A.; Kurenkova, A.; Kozlova, E. Photoinduced Deposition of Platinum from (Bu4N)2[Pt(NO3)6] for a Low Pt-Loading Pt/TiO2Hydrogen Photogeneration Catalyst. ACS Appl. Mater. Interfaces 2020, 12, 48631–48641. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, D.; Zhurenok, A.; Saraev, A.; Gerasimov, E.; Cherepanova, S.; Tkachev, S.; Plusnin, P.; Kozlova, E. Highly Efficient Hydrogen Production under Visible Light over G-C3N4-Based Photocatalysts with Low Platinum Content. Chem. Eng. J. 2022, 445, 136721. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Zhurenok, A.; Saraev, A.; Gerasimov, E.; Cherepanova, S.; Kovtunova, L.; Tkachev, S.; Kozlova, E. Platinum Deposition onto G-C3N4 with Using of Labile Nitratocomplex for Generation of the Highly Active Hydrogen Evolution Photocatalysts. Int. J. Hydrogen Energy 2022, 47, 11326–11340. [Google Scholar] [CrossRef]
- Berdyugin, S.N.; Vasilchenko, D.B.; Baidina, I.A.; Korenev, S.V.; Korolkov, I.V. Crystal Structure and Properties of [Rh2(H2O)8(μ-OH)2](NO3)4·4H2O. J. Struct. Chem. 2018, 59, 664–668. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Topchiyan, P.; Berdyugin, S.; Plyusnin, P.; Shayapov, V.; Baidina, I.; Komarov, V.; Bukhtiyarov, A.; Gerasimov, E. Tetranitratopalladate(II) Salts with Tetraalkylammonium Cations: Structural Aspects, Reactivity, and Applicability toward Palladium Deposition for Catalytic Applications. Inorg. Chem. 2021, 60, 2983–2995. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Yuan, Y.; Li, L.; Zhang, M.; Zhou, C.; Lin, Z. A Rapid Microwave-Assisted Thermolysis Route to Highly Crystalline Carbon Nitrides for Efficient Hydrogen Generation. Angew. Chemie 2016, 128, 14913–14917. [Google Scholar] [CrossRef]
- Vu, N.; Nguyen, C.; Kaliaguine, S.; Do, T. Synthesis of G-C3N4 Nanosheets by Using a Highly Condensed Lamellar Crystalline Melamine–Cyanuric Acid Supramolecular Complex for Enhanced Solar Hydrogen Generation. ChemSusChem 2019, 12, 291–302. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Wang, Z.; Jing, H.; Zhang, R. Microwave-Assisted Molten-Salt Rapid Synthesis of Isotype Triazine-/Heptazine Based g-C3N4 Heterojunctions with Highly Enhanced Photocatalytic Hydrogen Evolution Performance. Appl. Catal. B Environ. 2017, 203, 300–313. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Xiong, T.; Ni, Z.; Zhang, W.; Sun, Y.; Ho, W.-K. In Situ Construction of G-C3N4/g-C3N4 Metal-Free Heterojunction for Enhanced Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11392–11401. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.V.; Kaichev, V.V.; Saraev, A.A.; Gorodetskii, V.V.; Knop-Gericke, A.; Bukhtiyarov, V.I.; Nieuwenhuys, B.E. Oxidation of Propylene over Pd(5 5 1): Temperature Hysteresis Induced by Carbon Deposition and Oxygen Adsorption. Catal. Today 2015, 244, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of Sacrificial Reagents on Photocatalytic Hydrogen Evolution over Different Photocatalysts. J. Mater. Sci. 2017, 52, 5155–5164. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.; Badreldin, A.; Chava, R.; Do, J.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Pellegrin, Y.; Odobel, F. Les Donneurs d’électron Sacrificiels Pour La Production de Combustible Solaire. Comptes Rendus Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef] [Green Version]
- Vasilchenko, D.; Tkachenko, P.; Tkachev, S.; Popovetskiy, P.; Komarov, V.; Asanova, T.; Asanov, I.; Filatov, E.; Maximovskiy, E.; Gerasimov, E.; et al. Sulfuric Acid Solutions of [Pt(OH)4(H2O)2]: A Platinum Speciation Survey and Hydrated Pt(IV) Oxide Formation for Practical Use. Inorg. Chem. 2022, 61, 9667–9684. [Google Scholar] [CrossRef]









| No. | Photocatalyst | Metal Weight Content, % | Catalytic Activity, µmol gcat−1 h−1 | Catalytic Activity, mol gMetal−1 h−1 | AQY, % | Ref. |
|---|---|---|---|---|---|---|
| 1 | g-C3N4 | - | <1 | 0 | 0 | |
| Pd/g-C3N4 | ||||||
| 2 | Pd/g-C3N4 | 0.01 | 96 | 1.0 | <0.1 | This study |
| 3 | 0.05 | 620 | 1.2 | 0.4 | ||
| 4 | 0.1 | 1080 | 1.1 | 0.7 | ||
| 5 | 0.5 | 3100 | 0.6 | 1.8 | ||
| 6 | Pd/g-C3N4 (from [Pd(NH3)4(NO3)2]) | 0.5 | 1730 | 0.3 | 1.0 | |
| 7 | Pd/g-C3N4 (from PdCl2) | 0.5 | 3300 | 0.7 | - | |
| 8 | 0.5 | 7600 | 1.5 | 2.4 (400 nm) | [27] * | |
| Rh/g-C3N4 | ||||||
| 9 | Rh/g-C3N4 | 0.01 | 88 | 0.9 | <0.1 | This study |
| 10 | 0.05 | 732 | 1.5 | 0.4 | ||
| 11 | 0.1 | 2400 | 2.4 | 1.4 | ||
| 12 | 0.5 | 3900 | 0.8 | 2.3 | ||
| 13 | Rh/g-C3N4 (from RhCl3) | 0.5 | 610 | 0.1 | 0.4 | |
| 14 | Rh/g-C3N4 (from RhCl3) | 0.25 | 14.9 | 0.006 | - | [26] ** |
| 15 | 0.23 | 13.3 | 0.006 | |||
| 16 | Rh/g-C3N4 (from Rh(acac)3 | 0.34 | 10.7 | 0.003 | ||
| 17 | 0.27 | 3.8 | 0.001 | |||
| Pt/g-C3N4 | ||||||
| 18 | Pt0.1/g-C3N4 | 0.1 | 8520 | 8.5 | 5.0 | [35] *** |
| 19 | Pt0.5/g-C3N4 | 0.5 | 11,300 | 2.2 | 6.6 | |
| 20 | Pt0.1/g-C3N4 | 0.1 | 7560 | 7.6 | 4.4 | [47] *** |
| 21 | Pt0.5/g-C3N4 | 0.5 | 8520 | 1.7 | 5.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhurenok, A.V.; Vasichenko, D.B.; Berdyugin, S.N.; Gerasimov, E.Y.; Saraev, A.A.; Cherepanova, S.V.; Kozlova, E.A. Photocatalysts Based on Graphite-like Carbon Nitride with a Low Content of Rhodium and Palladium for Hydrogen Production under Visible Light. Nanomaterials 2023, 13, 2176. https://doi.org/10.3390/nano13152176
Zhurenok AV, Vasichenko DB, Berdyugin SN, Gerasimov EY, Saraev AA, Cherepanova SV, Kozlova EA. Photocatalysts Based on Graphite-like Carbon Nitride with a Low Content of Rhodium and Palladium for Hydrogen Production under Visible Light. Nanomaterials. 2023; 13(15):2176. https://doi.org/10.3390/nano13152176
Chicago/Turabian StyleZhurenok, Angelina V., Danila B. Vasichenko, Semen N. Berdyugin, Evgeny Yu. Gerasimov, Andrey A. Saraev, Svetlana V. Cherepanova, and Ekaterina A. Kozlova. 2023. "Photocatalysts Based on Graphite-like Carbon Nitride with a Low Content of Rhodium and Palladium for Hydrogen Production under Visible Light" Nanomaterials 13, no. 15: 2176. https://doi.org/10.3390/nano13152176