Eucalyptus globulus Mediated Green Synthesis of Environmentally Benign Metal Based Nanostructures: A Review
Abstract
:1. Introduction
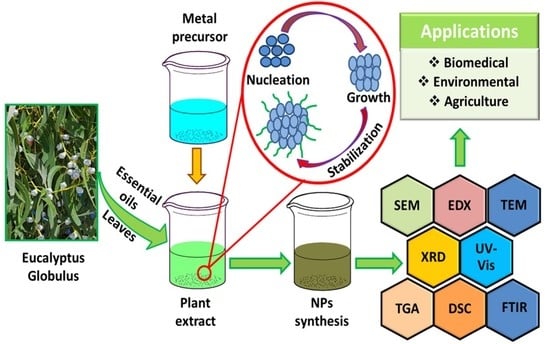
2. Plant Extract-Mediated Synthesis and Characterization of Nanoparticles
Characterization of Nanoparticles
3. Nanostructures Phytosynthesized Using Eucalyptus globulus Extract and Essential Oils
3.1. Zinc Oxide Nanoparticles (ZnO NPs)
3.2. Copper Oxide Nanoparticles
3.3. Magnesium Oxide Nanoparticles
3.4. Iron Oxide Nanoparticles
3.5. Lead Oxide Nanoparticles
3.6. Nickel Oxide Nanoparticles
3.7. Lanthanum Oxide Nanoparticles
3.8. Gold Nanoparticles
3.9. Silver Nanoparticles
3.10. Titanium Dioxide Nanoparticles
3.11. Zirconium Oxide Nanoparticles
4. The Probable Mechanism of Synthesis of Nanoparticles
5. Advantages and Challenges of Green Synthesis
6. Conclusions and Future Prospective
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| Ag | Silver |
| Au | Gold |
| A. mali | Alternaria mali |
| B. dothidea | Botryosphaeria dothidea |
| CuO | Copper oxide |
| DLS | Dynamic light scattering |
| DSC | Differential scanning calorimetry |
| D. seriata | Diplodia seriata |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| E. coli | Escherichia coli |
| EDX | Energy-dispersive X-ray spectroscopy |
| EG | Eucalyptus globulus |
| EOs | Essential oils |
| FTIR | Fourier transform infrared spectroscopy |
| K. leb | Klebsiella pneumonia |
| La2O3 | Lanthanum oxide |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NPs | Nanoparticles |
| P. aeruginosa | Pseudomonas aeruginosa |
| PbO | Lead oxide |
| SEM | Scanning electron microscopy |
| SPR | Surface plasmon resonance |
| TEM | Transmission electron microscopy |
| TFPH | Trifluoperazine dihydrochloride |
| TGA | Thermogravimetric analysis |
| TiO2 | Titanium dioxide |
| XPS | X-ray photoelectron spectroscopy |
| ZnO | Zinc oxide |
| ZrO2 | Zirconium oxide |
References
- Shume, W.M.; Murthy, H.A.; Zereffa, E.A. A review on synthesis and characterization of Ag2O nanoparticles for photocatalytic applications. J. Chem. 2020, 2020, 5039479. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Raj, A.; Tseng, T.H.; Xiao, H.; Nguyen, R.; Mohammed, F.S.; Halder, S.; Xu, M.; Wu, M.J.; Bao, S. Biocompatibility of nanomaterials and their immunological properties. Biomed. Mater. 2021, 16, 042005. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.S.S.; Estrella-Pajulas, L.L.; Alemaida, I.M.; Grilli, M.L.; Mikhaylov, A.; Senjyu, T. Green synthesis of silver oxide nanoparticles for photocatalytic environmental remediation and biomedical applications. Metals 2022, 12, 769. [Google Scholar] [CrossRef]
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus cup—A roman nanotechnology. Gold Bull. 2007, 40, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Khalaj, M.; Kamali, M.; Costa, M.E.V.; Capela, I. Green synthesis of nanomaterials—A scientometric assessment. J. Clean. Prod. 2020, 267, 122036. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- Kanwar, R.; Rathee, J.; Salunke, D.B.; Mehta, S.K. Green nanotechnology-driven drug delivery assemblies. ACS Omega 2019, 4, 8804–8815. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.; Sun, L.; Li, X.; Zhang, W.; Zhang, D.; Cai, J. Micro/Nanofabrication, Assembly, and Actuation Based on Microorganisms: Recent Advances and Perspectives. Small Struct. 2023, 2200356. [Google Scholar] [CrossRef]
- Herrera-Beurnio, M.C.; Hidalgo-Carrillo, J.; López-Tenllado, F.J.; Martin-Gómez, J.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Bio-templating: An emerging synthetic technique for catalysts. A review. Catalysts 2021, 11, 1364. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An overview of the algae-mediated biosynthesis of nanoparticles and their biomedical applications. Biomolecules 2020, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; Lima, R.D. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadoun, S.; Chauhan, N.P.S.; Zarrintaj, P.; Barani, M.; Varma, R.S.; Chinnam, S.; Rahdar, A. Synthesis of nanoparticles using microorganisms and their applications: A review. Environ. Chem. Lett. 2022, 20, 3153–3197. [Google Scholar] [CrossRef]
- Gong, D.; Celi, N.; Zhang, D.; Cai, J. Magnetic biohybrid microrobot multimers based on chlorella cells for enhanced targeted drug delivery. ACS Appl. Mater. Interfaces 2022, 14, 6320–6330. [Google Scholar] [CrossRef]
- Ahmed, A.; Usman, M.; Ji, Z.; Rafiq, M.; Yu, B.; Shen, Y.; Cong, H. Nature-inspired biogenic synthesis of silver nanoparticles for antibacterial applications. Mater. Today Chem. 2023, 27, 101339. [Google Scholar] [CrossRef]
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Mabood, Z.U.; Hano, C.; Abbasi, B.H. Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: A review. Int. J. Nanomed. 2020, 15, 5951–5961. [Google Scholar] [CrossRef] [PubMed]
- Rakib-Uz-Zaman, S.; Hoque Apu, E.; Muntasir, M.N.; Mowna, S.A.; Khanom, M.G.; Jahan, S.S.; Akter, N.; R. Khan, M.A.; Shuborna, N.S.; Shams, S.M. Biosynthesis of silver nanoparticles from Cymbopogon citratus leaf extract and evaluation of their antimicrobial properties. Challenges 2022, 13, 18. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Oves, M.; Rauf, M.A.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Zaman, G.S.; Saeed, M. Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Hadi, R.; Tahmasebi, B.; Farhoudian, S.; Mehravar, M.; Nasiri, R. Green synthesis of gold nanoparticles using plant extract: Mini-review. Nanochem. Res. 2017, 2, 8–19. [Google Scholar]
- Santhosh, P.B.; Genova, J.; Chamati, H. Green synthesis of gold nanoparticles: An eco-friendly approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A review on green synthesis of nanoparticles and their diverse biomedical and environmental applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Ridolfo, R.; Tavakoli, S.; Junnuthula, V.; Williams, D.S.; Urtti, A.; van Hest, J.C. Exploring the impact of morphology on the properties of biodegradable nanoparticles and their diffusion in complex biological medium. Biomacromolecules 2020, 22, 126–133. [Google Scholar] [CrossRef]
- Rajendran, N.K.; George, B.P.; Houreld, N.N.; Abrahamse, H. Synthesis of zinc oxide nanoparticles using Rubus fairholmianus root extract and their activity against pathogenic bacteria. Molecules 2021, 26, 3029. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Jian, N.; Dowle, M.; Horniblow, R.D.; Tselepis, C.; Palmer, R.E. Morphology of the ferritin iron core by aberration corrected scanning transmission electron microscopy. Nanotechnology 2016, 27, 46LT02. [Google Scholar] [CrossRef]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B 2016, 162, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Zeng, Y.; Wang, G.; Han, S.; Yang, Z.; Li, B.; Wang, X.; Gao, J.; Zheng, L. Leucine-coated cobalt ferrite nanoparticles: Synthesis, characterization and potential biomedical applications for drug delivery. Phys. Lett. A 2020, 384, 126600. [Google Scholar] [CrossRef]
- Grattapaglia, D.; Vaillancourt, R.E.; Shepherd, M.; Thumma, B.R.; Foley, W.; Külheim, C.; Potts, B.M.; Myburg, A.A. Progress in Myrtaceae genetics and genomics: Eucalyptus as the pivotal genus. Tree Genet. Genomes 2012, 8, 463–508. [Google Scholar] [CrossRef] [Green Version]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C. Chemical composition and effect against skin alterations of bioactive extracts obtained by the hydrodistillation of Eucalyptus globulus leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, M. Bioactive potential of essential oil extracted from the leaves of Eucalyptus globulus (Myrtaceae). J. Pharmacogn. Phytochem. 2019, 8, 213–216. [Google Scholar]
- James, S.A.; Bell, D.T. Leaf morphological and anatomical characteristics of heteroblastic Eucalyptus globulus ssp. globulus (Myrtaceae). Aust. J. Bot. 2001, 49, 259–269. [Google Scholar] [CrossRef]
- Bilar, A.; Christopher, B. Investigation of the inhibitive effect of aqueous extract of eucalyptus leaves on mild steel in acidic media. J. Chem. Soc. Niger. 2020, 45, 890–896. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiata essential oils. Ind. Crops Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Said, Z.B.-O.S.; Haddadi-Guemghar, H.; Boulekbache-Makhlouf, L.; Rigou, P.; Remini, H.; Adjaoud, A.; Khoudja, N.K.; Madani, K. Essential oils composition, antibacterial and antioxidant activities of hydrodistillated extract of Eucalyptus globulus fruits. Ind. Crops Prod. 2016, 89, 167–175. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Abbasi, N.; Khalighi, Z.; Eftekhari, Z.; Bahmani, M. Extraction and phytoanalysis of chemical compounds of Eucalyptus globulus leaf native to Dehloran, Ilam province, Iran by HS-SPME and GC-MS. Adv. Anim. Vet. Sci. 2020, 8, 647–652. [Google Scholar] [CrossRef]
- Dellacassa, E.; Menéndez, P.; Moyna, P.; Soler, E. Chemical composition of Eucalyptus essential oils grown in Uruguay. Flavour. Fragr. J. 1990, 5, 91–95. [Google Scholar] [CrossRef]
- Golestani, M.R.; Rad, M.; Bassami, M.; Afkhami-Goli, A. Analysis and evaluation of antibacterial effects of new herbal formulas, AP-001 and AP-002, against Escherichia coli O157: H7. Life Sci. 2015, 135, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Viturro, C.I.; Molina, A.C.; Heit, C.I. Volatile components of Eucalyptus globulus Labill ssp. bicostata from Jujuy, Argentina. J. Essent. Oil Res. 2003, 15, 206–208. [Google Scholar] [CrossRef]
- Aldoghaim, F.S.; Flematti, G.R.; Hammer, K.A. Antimicrobial activity of several cineole-rich Western Australian Eucalyptus essential oils. Microorganisms 2018, 6, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, A.; Sharma, A.; Bachheti, R.; Pandey, D. A comparative study of the chemical composition of the essential oil from Eucalyptus globulus growing in Dehradun (India) and around the world. Orient. J. Chem. 2016, 32, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Achmad, H.N.; Rana, H.E.; Fadilla, I.; Fajar, A.; Manurung, R.; Abduh, M.Y. Determination of yield, productivity and chemial composition of eucalyptus oil from different species and locations in indonesia. Biol. Nat. Resour. Eng. J. 2018, 1, 36–49. [Google Scholar]
- Damjanović-Vratnica, B.; Đakov, T.; Suković, D.; Damjanović, J. Antimicrobial effect of essential oil isolated from Eucalyptus globulus Labill. from Montenegro. Czech J. Food Sci. 2011, 29, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Almas, I.; Innocent, E.; Machumi, F.; Kisinza, W. Chemical composition of essential oils from Eucalyptus globulus and Eucalyptus maculata grown in Tanzania. Sci. Afr. 2021, 12, 00758. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Djenane, D.; Yangüela, J.; Amrouche, T.; Boubrit, S.; Boussad, N.; Roncalés, P. Chemical composition and antimicrobial effects of essential oils of Eucalyptus globulus, Myrtus communis and Satureja hortensis against Escherichia coli O157: H7 and Staphylococcus aureus in minced beef. Food Sci. Technol. Int. 2011, 17, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Djelloul, R.; Mokrani, K.; Hacini, N. Study of the antibacterial activity of the extract from the essential oil of Eucalyptus globulus and Rosmarinus officinalis on three bacterial strains. Int. J. Appl. Environ. Sci 2017, 12, 47–56. [Google Scholar]
- Boulekbache-Makhlouf, L.; Slimani, S.; Madani, K. Total phenolic content, antioxidant and antibacterial activities of fruits of Eucalyptus globulus cultivated in Algeria. Ind. Crops Prod. 2013, 41, 85–89. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.Z.; Yang, H.; Liu, H.Y.; An, L.K. Robustadial A and B from Eucalyptus globulus Labill. and their anticancer activity as selective tyrosyl-DNA phosphodiesterase 2 inhibitors. Phytother. Res. 2021, 35, 5282–5289. [Google Scholar] [CrossRef]
- Khazraei, H.; Shamsdin, S.A.; Zamani, M. In Vitro cytotoxicity and apoptotic assay of Eucalyptus globulus essential oil in colon and liver cancer cell lines. J. Gastrointest. Cancer 2021, 53, 363–369. [Google Scholar] [CrossRef]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef]
- Palma, A.; Díaz, M.J.; Ruiz-Montoya, M.; Morales, E.; Giráldez, I. Ultrasound extraction optimization for bioactive molecules from Eucalyptus globulus leaves through antioxidant activity. Ultrason. Sonochem. 2021, 76, 105654. [Google Scholar] [CrossRef]
- Bencheikh, D.; Gueddah, A.; Soualat, K.; Ben-aissi, H.; Benslama, A.; Harrar, A.; Khennouf, S. Polyphenolic contents, antioxidant and antibacterial activities of aqueous extracts of Eucalyptus globulus L. and Trigonella foenum-greacum L. J. Appl. Biol. Sci. 2021, 15, 53–63. [Google Scholar]
- Akolade, J.O.; Olajide, O.O.; Afolayan, M.O.; Akande, S.A.; Idowu, D.I.; Orishadipe, A.T. Chemical composition, antioxidant and cytotoxic effects of Eucalyptus globulus grown in north-central Nigeria. J. Nat. Prod. Plant. Resour. 2012, 2, 1–8. [Google Scholar]
- Quatrin, P.M.; Verdi, C.M.; de Souza, M.E.; de Godoi, S.N.; Klein, B.; Gundel, A.; Wagner, R.; de Almeida Vaucher, R.; Ourique, A.F.; Santos, R.C.V. Antimicrobial and antibiofilm activities of nanoemulsions containing Eucalyptus globulus oil against Pseudomonas aeruginosa and Candida spp. Microb. Pathogen. 2017, 112, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Bogavac, M.; Tešanović, K.; Marić, J.; Jovanović, M.; Karaman, M. Antimicrobial activity and toxicity of Eucalyptus globulus Labill. essential oil against vaginal microorganisms. Trends Phytochem. Res. 2019, 3, 201–206. [Google Scholar]
- Hafsa, J.; ali Smach, M.; Khedher, M.R.B.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Göger, G.; Karaca, N.; BÜYÜKKILIÇ, B.; Demirci, B.; Demirci, F. In vitro antimicrobial, antioxidant and anti-inflammatory evaluation of Eucalyptus globulus essential oil. Nat. Volatiles Essent. Oils 2020, 7, 1–11. [Google Scholar]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Ajilore, B.S.; Oluwadairo, T.O.; Olorunnisola, O.S.; Fadahunsi, O.S.; Adegbola, P.I. GC–MS analysis, toxicological and oral glucose tolerance assessments of methanolic leaf extract of Eucalyptus globulus. Future J. Pharm. Sci. 2021, 7, 162. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic properties of the terpenoids aromadendrene and 1, 8-cineole from the essential oil of Eucalyptus globulus against antibiotic-susceptible and antibiotic-resistant pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Emran, T.B.; Cavalu, S. Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.; Abdulkareem, A.; Tijani, J.; Shuaib, D.; Ajala, A.; Mohammed, A. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S. A review on green synthesis of zinc oxide nanoparticles using plant extracts and its biomedical applications. BioNanoScience 2020, 10, 848–863. [Google Scholar] [CrossRef]
- Siripireddy, B.; Mandal, B.K. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv. Powder Technol. 2017, 28, 785–797. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Dhanjal, D.S.; Sharma, A.; Nepovimova, E.; Kalia, A.; Thakur, S.; Bhardwaj, S.; Chopra, C.; Singh, R.; Verma, R. Conifer-derived metallic nanoparticles: Green synthesis and biological applications. Int. J. Mol. Sci. 2020, 21, 9028. [Google Scholar] [CrossRef]
- Shahid, S.; Fatima, U.; Sajjad, R.; Khan, S. Bioinspired nanotheranostic agent: Zinc oxide; green synthesis and biomedical potential. Dig. J. Nanomater. Biostruct. 2019, 14, 1023–1031. [Google Scholar]
- Barzinjy, A.A.; Azeez, H.H. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl. Sci. 2020, 2, 991. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeizi, Z.; Benbouzid, H.; Ouchenane, S.; Yılmaz, D.; Culha, M.; Bououdina, M. Biosynthesis of Zinc oxide nanoparticles from essential oil of Eucalyptus globulus with antimicrobial and anti-biofilm activities. Mater. Today Commun. 2020, 25, 101553. [Google Scholar] [CrossRef]
- Razanamahandry, L.; Sackey, J.; Furqan, C.; Ntwampe, S.; Fosso-Kankeu, E.; Manikandan, E.; Maaza, M. Removal of Free Cyanide by a Green Photocatalyst ZnO Nanoparticle Synthesized via Eucalyptus globulus Leaves. In Photocatalysts in Advanced Oxidation Processes for Wastewater Treatment; Fosso-Kankeu, E., Pandey, S., Sinha Ray, S., Eds.; Srinevener Publishing, Wiley: Beverley, UK, 2020; pp. 271–288. [Google Scholar]
- Masood, K.; Yasmin, H.; Batool, S.; Ilyas, N.; Nosheen, A.; Naz, R.; Khan, N.; Hassan, M.N.; Aldhahrani, A.; Althobaiti, F. A strategy for mitigating avian colibacillosis disease using plant growth promoting rhizobacteria and green synthesized zinc oxide nanoparticles. Saudi J. Biol. Sci. 2021, 28, 4957–4968. [Google Scholar] [CrossRef]
- Siddique, M.A.; Hasan, M.U.; Sagheer, M.; Sahi, S.T. Comparative toxic effects of Eucalyptus globulus L.(Myrtales: Myrtaceae) and its green synthesized zinc oxide nanoparticles (ZnONPs) against Rhyzopertha dominica (F.)(Coleoptera: Bostrichidae). Int. J. Trop. Insect Sci. 2022, 42, 1697–1706. [Google Scholar] [CrossRef]
- Hafeez, M.; Ghazal, A.; Khan, J.; Ahmad, P.; Khandaker, M.U.; Osman, H.; Alamri, S. Eucalyptus globulus Extract-Assisted Fabrication of Copper Oxide/Zinc Oxide Nanocomposite for Photocatalytic Applications. Crystals 2022, 12, 1153. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, N. Synthesis and biomedical applications of copper oxide nanoparticles: An expanding horizon. ACS Biomater. Sci. Eng. 2019, 5, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Waris, A.; Din, M.; Ali, A.; Ali, M.; Afridi, S.; Baset, A.; Khan, A.U. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg. Chem. Commun. 2021, 123, 108369. [Google Scholar] [CrossRef]
- Sundar, S.; Venkatachalam, G.; Kwon, S.J. Biosynthesis of copper oxide (CuO) nanowires and their use for the electrochemical sensing of dopamine. Nanomaterials 2018, 8, 823. [Google Scholar] [CrossRef] [Green Version]
- Benrezgua, E.; Deghfel, B.; Zoukel, A.; Basirun, W.J.; Amari, R.; Boukhari, A.; Yaakob, M.K.; Kheawhom, S.; Mohamad, A.A. Synthesis and properties of copper doped zinc oxide thin films by sol-gel, spin coating and dipping: A characterization review. J. Mol. Struct. 2022, 1267, 133639. [Google Scholar] [CrossRef]
- Maqbool, Q.; Iftikhar, S.; Nazar, M.; Abbas, F.; Saleem, A.; Hussain, T.; Kausar, R.; Anwaar, S.; Jabeen, N. Green fabricated CuO nanobullets via Olea europaea leaf extract shows auspicious antimicrobial potential. IET Nanobiotechnol. 2017, 11, 463–468. [Google Scholar] [CrossRef]
- Pinto, R.J.; Lucas, J.M.; Silva, F.M.; Girão, A.V.; Oliveira, F.J.; Marques, P.A.; Freire, C.S. Bio-based synthesis of oxidation resistant copper nanowires using an aqueous plant extract. J. Clean. Prod. 2019, 221, 122–131. [Google Scholar] [CrossRef]
- Ali, K.; Ahmed, B.; Ansari, S.M.; Saquib, Q.; Al-Khedhairy, A.A.; Dwivedi, S.; Alshaeri, M.; Khan, M.S.; Musarrat, J. Comparative in situ ROS mediated killing of bacteria with bulk analogue, Eucalyptus leaf extract (ELE)-capped and bare surface copper oxide nanoparticles. Mater. Sci. Eng. C 2019, 100, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Alhalili, Z. Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract: Adsorption and design of experiments. Arab. J. Chem. 2022, 15, 103739. [Google Scholar] [CrossRef]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Hornak, J. Synthesis, properties, and selected technical applications of magnesium oxide nanoparticles: A review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Shen, Y.; He, L.; Yang, Z.; Xiong, Y. Corrosion behavior of different coatings prepared on the surface of AZ80 magnesium alloy in simulated body fluid. J. Mater. Eng. Perform. 2020, 29, 1609–1621. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Klapiszewski, Ł.; Jesionowski, T. Recent development in the synthesis, modification and application of Mg (OH)2 and MgO: A review. Powder Technol. 2017, 319, 373–407. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Hornak, J.; Trnka, P.; Kadlec, P.; Michal, O.; Mentlík, V.; Šutta, P.; Csányi, G.M.; Tamus, Z.Á. Magnesium oxide nanoparticles: Dielectric properties, surface functionalization and improvement of epoxy-based composites insulating properties. Nanomaterials 2018, 8, 381. [Google Scholar] [CrossRef] [Green Version]
- Jeevanandam, J.; Chan, Y.S.; Ku, Y.H. Aqueous Eucalyptus globulus leaf extract-mediated biosynthesis of MgO nanorods. Appl. Biol. Chem. 2018, 61, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [Green Version]
- Hasany, S.; Ahmed, I.; Rajan, J.; Rehman, A. Systematic review of the preparation techniques of iron oxide magnetic nanoparticles. Nanosci. Nanotechnol. 2012, 2, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Balamurugan, M.; Saravanan, S.; Soga, T. Synthesis of iron oxide nanoparticles by using Eucalyptus globulus plant extract. e-J. Surf. Sci. Nanotechnol. 2014, 12, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Zavaleta, K.; Chacon-Laiza, Y.; Asmat-Campos, D.; Raquel-Checca, N. Green synthesis of superparamagnetic iron oxide nanoparticles with Eucalyptus globulus extract and their application in the removal of heavy metals from agricultural soil. Molecules 2022, 27, 1367. [Google Scholar] [CrossRef] [PubMed]
- Nazaripour, E.; Mousazadeh, F.; Moghadam, M.D.; Najafi, K.; Borhani, F.; Sarani, M.; Ghasemi, M.; Rahdar, A.; Iravani, S.; Khatami, M. Biosynthesis of lead oxide and cerium oxide nanoparticles and their cytotoxic activities against colon cancer cell line. Inorg. Chem. Commun. 2021, 131, 108800. [Google Scholar] [CrossRef]
- Miri, A.; Sarani, M.; Hashemzadeh, A.; Mardani, Z.; Darroudi, M. Biosynthesis and cytotoxic activity of lead oxide nanoparticles. Green Chem. Lett. Rev. 2018, 11, 567–572. [Google Scholar] [CrossRef]
- Noukelag, S.; Mohamed, H.; Razanamahandry, L.; Ntwampe, S.; Arendse, C. Bio-inspired synthesis of PbO nanoparticles (NPs) via an aqueous extract of Rosmarinus officinalis (rosemary) leaves. Mater. Today Proc. 2021, 36, 421–426. [Google Scholar] [CrossRef]
- Muhammad, W.; Khan, M.A.; Nazir, M.; Siddiquah, A.; Mushtaq, S.; Hashmi, S.S.; Abbasi, B.H. Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mater. Sci. Eng. C 2019, 103, 109740. [Google Scholar] [CrossRef]
- Elango, G.; Roopan, S.M. Green synthesis, spectroscopic investigation and photocatalytic activity of lead nanoparticles. Spectrochim. Acta Part A 2015, 139, 367–373. [Google Scholar] [CrossRef]
- Tailor, G.; Lawal, A. Phytochemical screening; green synthesis, characterization and biological significance of lead oxide nanoparticles from Eucalyptus globulus Labill.(leaves). Nanotechnol. Environ. Eng. 2021, 6, 48. [Google Scholar] [CrossRef]
- Paulose, R.; Mohan, R.; Parihar, V. Nanostructured nickel oxide and its electrochemical behaviour—A brief review. Nano-Struct. Nano-Objects 2017, 11, 102–111. [Google Scholar] [CrossRef]
- Ahmad, W.; Bhatt, S.C.; Verma, M.; Kumar, V.; Kim, H. A review on current trends in the green synthesis of nickel oxide nanoparticles, characterizations, and their applications. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100674. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Huang, D.; Chen, X.; Zhu, J.; Sun, D.; Huang, J.; Li, Q. Plant-mediated synthesis of size-controllable Ni nanoparticles with alfalfa extract. Mater. Lett. 2014, 122, 166–169. [Google Scholar] [CrossRef]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathogen. 2017, 111, 375–387. [Google Scholar] [CrossRef]
- Sovizi, M.R.; Mirzakhani, S. A chemiresistor sensor modified with lanthanum oxide nanoparticles as a highly sensitive and selective sensor for dimethylamine at room temperature. New J. Chem. 2020, 44, 4927–4934. [Google Scholar] [CrossRef]
- Madani, R.F.; Sofianty, I.; Sari, A.G.P.; Maryanti, R.; Nandiyanto, A.B.D. Synthesis methods and green synthesis of lanthanum oxide nanoparticles: A review. Arab. J. Chem. Environ. Res. 2021, 8, 287–314. [Google Scholar]
- Maheshwaran, G.; Muneeswari, R.S.; Bharathi, A.N.; Kumar, M.K.; Sudhahar, S. Eco-friendly synthesis of lanthanum oxide nanoparticles by Eucalyptus globulus leaf extracts for effective biomedical applications. Mater. Lett. 2021, 283, 128799. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef] [Green Version]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical applications of functionalized gold nanoparticles: A review. J. Clust. Sci. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Khan, T.; Ullah, N.; Khan, M.A.; Nadhman, A. Plant-based gold nanoparticles; A comprehensive review of the decade-long research on synthesis, mechanistic aspects and diverse applications. Adv. Colloid Interface Sci. 2019, 272, 102017. [Google Scholar] [CrossRef] [PubMed]
- Hassanisaadi, M.; Bonjar, G.H.S.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally safe biosynthesis of gold nanoparticles using plant water extracts. Nanomaterials 2021, 11, 2033. [Google Scholar] [CrossRef]
- Pinto, R.J.; Lucas, J.M.; Morais, M.P.; Santos, S.A.; Silvestre, A.J.; Marques, P.A.; Freire, C.S. Demystifying the morphology and size control on the biosynthesis of gold nanoparticles using Eucalyptus globulus bark extract. Ind. Crops Prod. 2017, 105, 83–92. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Berent, S.; Motyka, A.; Jamroz, P.; Kurcbach, K.; Sledz, W.; Pohl, P. Comparison of the characteristics of gold nanoparticles synthesized using aqueous plant extracts and natural plant essential oils of Eucalyptus globulus and Rosmarinus officinalis. Arab. J. Chem. 2019, 12, 4795–4805. [Google Scholar] [CrossRef] [Green Version]
- Ibraheem, I.J.; Hassan, K.T.; Ali, H.H.; Obaid, A.S. One-Step Biosynthesis of Gold Nanoparticles Using Natural Reductive Extracts. Nano Biomed. Eng. 2020, 12, 331–337. [Google Scholar] [CrossRef]
- Astalakshmi, A.; Nima, P.; Ganesan, V. A green approach in the synthesis of silver nanoparticles using bark of Eucalyptus globulus, Labill. Int. J. Pharm. Sci. Res. 2013, 23, 47–52. [Google Scholar]
- Ali, K.; Ahmed, B.; Dwivedi, S.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Microwave accelerated green synthesis of stable silver nanoparticles with Eucalyptus globulus leaf extract and their antibacterial and antibiofilm activity on clinical isolates. PLoS ONE 2015, 10, e0131178. [Google Scholar] [CrossRef]
- Balamurugan, M.; Saravanan, S. Green synthesis of silver nanoparticles by using Eucalyptus globulus leaf extract. J. Inst. Eng. India Ser. A 2017, 98, 461–467. [Google Scholar] [CrossRef]
- Balčiūnaitienė, A.; Liaudanskas, M.; Puzerytė, V.; Viškelis, J.; Janulis, V.; Viškelis, P.; Griškonis, E.; Jankauskaitė, V. Eucalyptus globulus and Salvia officinalis extracts mediated green synthesis of silver nanoparticles and their application as an antioxidant and antimicrobial agent. Plants 2022, 11, 1085. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef] [PubMed]
- Mollavali, M.; Falamaki, C.; Rohani, S. Efficient light harvesting by NiS/CdS/ZnS NPs incorporated in C, N-co-doped-TiO2 nanotube arrays as visible-light sensitive multilayer photoanode for solar applications. Int. J. Hydrogen Energy 2018, 43, 9259–9278. [Google Scholar] [CrossRef]
- Narkevica, I.; Stradina, L.; Stipniece, L.; Jakobsons, E.; Ozolins, J. Electrophoretic deposition of nanocrystalline TiO2 particles on porous TiO2-X ceramic scaffolds for biomedical applications. J. Eur. Ceram. Soc. 2017, 37, 3185–3193. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. TiO2 NPs assembled into a carbon nanofiber composite electrode by a one-step electrospinning process for supercapacitor applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Ansari, A.; Siddiqui, V.U.; Rehman, W.U.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Green synthesis of TiO2 nanoparticles using Acorus calamus leaf extract and evaluating its photocatalytic and in vitro antimicrobial activity. Catalysts 2022, 12, 181. [Google Scholar] [CrossRef]
- Nabi, G.; Ain, Q.U.; Tahir, M.B.; Nadeem Riaz, K.; Iqbal, T.; Rafique, M.; Hussain, S.; Raza, W.; Aslam, I.; Rizwan, M. Green synthesis of TiO2 nanoparticles using lemon peel extract: Their optical and photocatalytic properties. Int. J. Environ. Anal. Chem. 2022, 102, 434–442. [Google Scholar] [CrossRef]
- Balaji, S.; Guda, R.; Mandal, B.K.; Kasula, M.; Ubba, E.; Khan, F.-R.N. Green synthesis of nano-titania (TiO2 NPs) utilizing aqueous Eucalyptus globulus leaf extract: Applications in the synthesis of 4 H-pyran derivatives. Res. Chem. Intermed. 2021, 47, 3919–3931. [Google Scholar] [CrossRef]
- Torres-Limiñana, J.; Feregrino-Pérez, A.A.; Vega-González, M.; Escobar-Alarcón, L.; Cervantes-Chávez, J.A.; Esquivel, K. Green synthesis via Eucalyptus globulus L. extract of Ag-TiO2 catalyst: Antimicrobial activity evaluation toward water disinfection process. Nanomaterials 2022, 12, 1944. [Google Scholar] [CrossRef]
- Nikam, A.; Pagar, T.; Ghotekar, S.; Pagar, K.; Pansambal, S. A review on plant extract mediated green synthesis of zirconia nanoparticles and their miscellaneous applications. J. Chem. Rev. 2019, 1, 154–163. [Google Scholar]
- Su, Y.; Cui, H.; Li, Q.; Gao, S.; Shang, J.K. Strong adsorption of phosphate by amorphous zirconium oxide nanoparticles. Water Res. 2013, 47, 5018–5026. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Szostak, K.; Banach, M. Photocatalytic properties of zirconium oxide–zinc oxide nanoparticles synthesised using microwave irradiation. Appl. Nanosci. 2020, 10, 941–954. [Google Scholar] [CrossRef] [Green Version]
- Balaji, S.; Mandal, B.K.; Ranjan, S.; Dasgupta, N.; Chidambaram, R. Nano-zirconia–evaluation of its antioxidant and anticancer activity. J. Photochem. Photobiol. B 2017, 170, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.P.; Li, S.F.Y. Potential of plant as a biological factory to synthesize gold and silver nanoparticles and their applications. Rev. Environ. Sci. Bio/Technol. 2012, 11, 169–206. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Sajjad, W.; Wu, F.; Bibi, N.; Shah, K.; Yali, Z.; Wang, W. Green synthesis of silver nanoparticles and their shortcomings, animal blood a potential source for silver nanoparticles: A review. J. Hazard. Mater. Adv. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pagar, T.; Pansambal, S.; Oza, R. A review on green synthesis of sulfur nanoparticles via plant extract, characterization and its applications. Adv. J. Chem. Sect. B 2020, 2, 128–143. [Google Scholar]
- Mondal, P.; Anweshan, A.; Purkait, M.K. Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: A review. Chemosphere 2020, 259, 127509. [Google Scholar] [CrossRef]
- Soltys, L.; Olkhovyy, O.; Tatarchuk, T.; Naushad, M. Green synthesis of metal and metal oxide nanoparticles: Principles of green chemistry and raw materials. Magnetochemistry 2021, 7, 145. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. Sci. Res. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Aboyewa, J.A.; Sibuyi, N.R.; Meyer, M.; Oguntibeju, O.O. Green synthesis of metallic nanoparticles using some selected medicinal plants from southern africa and their biological applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Kumari, P.; Bontempi, E.; Yadav, S. Medicinal plants: Treasure trove for green synthesis of metallic nanoparticles and their biomedical applications. Biocatal. Agric. Biotechnol. 2020, 24, 101518. [Google Scholar] [CrossRef]
- Pal, K.; Chakroborty, S.; Nath, N. Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications. Green Process. Synth. 2022, 11, 951–964. [Google Scholar] [CrossRef]
- Shanker, U.; Jassal, V.; Rani, M.; Kaith, B.S. Towards green synthesis of nanoparticles: From bio-assisted sources to benign solvents. A review. Int. J. Environ. Anal. Chem. 2016, 96, 801–835. [Google Scholar]
- Schons, A.B.; Appelt, P.; Correa, J.S.; Cunha, M.A.; Rodrigues, M.G.; Anaissi, F.J. Green Synthesis of Na abietate Obtained from the Salification of Pinus elliottii Resin with Promising Antimicrobial Action. Antibiotics 2023, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.M.M.; Ahmed, S.M.; Abdulrahman, A.F.; AlMessiere, M.A. Characterization of green synthesized of ZnO nanoparticles by using pinus brutia leaves extracts. J. Mol. Struct. 2023, 1280, 135063. [Google Scholar] [CrossRef]
- Satpathy, S.; Patra, A.; Ahirwar, B.; Hussain, M.D. Process optimization for green synthesis of gold nanoparticles mediated by extract of Hygrophila spinosa T. Anders and their biological applications. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 121, 113830. [Google Scholar] [CrossRef]












| Pharmacological Activity | Method Used | Plant Parts | Reference |
|---|---|---|---|
| Antibacterial | Agar diffusion method, Disc diffusion method | Leaves, ariel part, fruit | [35,39,52,53,54] |
| Anticancer | MMT assay | Fruit, essential oils | [55,56] |
| Antioxidant | DPPH assay, ABTS method | Leaves | [39,57,58,59,60] |
| Antifungal | Broth microdilution assay, double dilution micro-plate assay, disc diffusion method | Leaves | [49,61,62,63,64] |
| Neuro-protective | MMT assay | Leaves | [57,65] |
| Anti-hyperglycemic | Monitored oral glucose tolerance test | Leaves | [66] |
| Plant Parts | Significance | Morphology | Size (nm) | Reference |
|---|---|---|---|---|
| Leaves | Photocatalytic and antioxidant activities | Spherical and hexagonal | 10–20 | [74] |
| Leaves | Antibacterial, antioxidant, anticancer activities | Spherical | 10–30 | [78] |
| Leaves | -- | Hexagonal | 35 | [79] |
| Leaves | Antifungal activity | Agglomerated clusters | 52–70 | [80] |
| Essential oils | Antimicrobial and anti-biofilm activities | Spherical and needle-like | 40 | [81] |
| Leaves | Photocatalytic activity | Spherical | -- | [82] |
| Leaves | Antibacterial activity | -- | -- | [83] |
| Leaves | Antimicrobial activity | Spherical, flower, and walnut shape | 186.7 | [84] |
| Leaves | Photocatalytic activity | Agglomerated shape | -- | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadiq, M.U.; Shah, A.; Haleem, A.; Shah, S.M.; Shah, I. Eucalyptus globulus Mediated Green Synthesis of Environmentally Benign Metal Based Nanostructures: A Review. Nanomaterials 2023, 13, 2019. https://doi.org/10.3390/nano13132019
Sadiq MU, Shah A, Haleem A, Shah SM, Shah I. Eucalyptus globulus Mediated Green Synthesis of Environmentally Benign Metal Based Nanostructures: A Review. Nanomaterials. 2023; 13(13):2019. https://doi.org/10.3390/nano13132019
Chicago/Turabian StyleSadiq, Muhammad Usman, Afzal Shah, Abdul Haleem, Syed Mujtaba Shah, and Iltaf Shah. 2023. "Eucalyptus globulus Mediated Green Synthesis of Environmentally Benign Metal Based Nanostructures: A Review" Nanomaterials 13, no. 13: 2019. https://doi.org/10.3390/nano13132019