Wood Cellulose Nanofibers Grafted with Poly(ε-caprolactone) Catalyzed by ZnEu-MOF for Functionalization and Surface Modification of PCL Films
Abstract
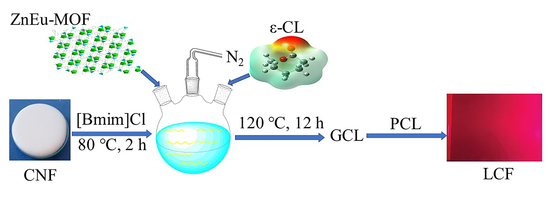
:1. Introduction
2. Experiments and Methods
2.1. Materials
2.2. Synthesis of the ZnEu-MOF
2.3. Synthesis of GCL
2.4. Preparation of PCL and the Composite Films
2.5. Characterization
2.6. In Vitro Cytotoxicity
2.7. Computational methods
3. Result and Discussion
3.1. Characterization of ZnEu-MOF
3.2. Characterization of GCL and Catalytic Synthesis Mechanism
3.3. Characterization of PCL and the Composite Films
3.3.1. Macroscopic Mechanical Properties and Morphology
3.3.2. Physical-Chemical Properties of PCL and the Composite Films
3.3.3. Fluorescent Properties of the Composite Films
3.4. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Li, L.; Nishiyama, Y.; Reid, M.S.; Berglund, L.A. Processing Strategy for Reduced Energy Demand of Nanostructured CNF/Clay Composites with Tailored Interfaces. Carbohydr. Polym. 2023, 312, 120788. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial Bio-Nanocomposites and Their Potential Applications in Food Packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Park, S.Y.; Yook, S.; Goo, S.; Im, W.; Youn, H.J. Preparation of Transparent and Thick CNF/Epoxy Composites by Controlling the Properties of Cellulose Nanofibrils. Nanomaterials 2020, 10, 625. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Jeon, B.; Bae, J.H.; Kim, I.S.; Paeng, K.; Kim, M.; Lee, H. Thiol-Based Chemistry as Versatile Routes for the Effective Functionalization of Cellulose Nanofibers. Carbohydr. Polym. 2019, 226, 115259. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, B.; da Costa, M.V.T.; Dai, H.; Ram, F.; Li, Y. ZnO Microrods Sandwiched between Layered CNF Matrix: Fabrication, Stress Transfer, and Mechanical Properties. Carbohydr. Polym. 2023, 305, 120536. [Google Scholar] [CrossRef] [PubMed]
- Ishak, A.; Sonnier, R.; Otazaghine, B.; Longuet, C. Silazanes, a Novel Flax Fibers Functionalization: Effect on Silicone-Based Composites. Compos. Part A Appl. Sci. Manuf. 2023, 166, 107382. [Google Scholar] [CrossRef]
- Cesari, A.; Loureiro, M.V.; Vale, M.; Yslas, E.I.; Dardanelli, M.; Marques, A.C. Polycaprolactone Microcapsules Containing Citric Acid and Naringin for Plant Growth and Sustainable Agriculture: Physico-Chemical Properties and Release Behavior. Sci. Total Environ. 2020, 703, 135548. [Google Scholar] [CrossRef]
- Corrêa, A.C.; de Campos, A.; Claro, P.I.C.; Guimarães, G.G.F.; Mattoso, L.H.C.; Marconcini, J.M. Biodegradability and Nutrients Release of Thermoplastic Starch and Poly(ε-caprolactone) Blends for Agricultural Uses. Carbohydr. Polym. 2022, 282, 119058. [Google Scholar] [CrossRef]
- Grillo, R.; dos Santos, N.Z.P.; Maruyama, C.R.; Rosa, A.H.; de Lima, R.; Fraceto, L.F. Poly(ε-caprolactone) Nanocapsules as Carrier Systems for Herbicides: Physico-Chemical Characterization and Genotoxicity Evaluation. J. Hazard. Mater. 2012, 231–232, 1–9. [Google Scholar] [CrossRef]
- Mahmoud, L.A.M.; Telford, R.; Livesey, T.C.; Katsikogianni, M.; Kelly, A.L.; Terry, L.R.; Ting, V.P.; Nayak, S. Zirconium-Based Mofs and Their Biodegradable Polymer Composites for Controlled and Sustainable Delivery of Herbicides. ACS Appl. Bio Mater. 2022, 5, 3972–3981. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, A.; Fang, D.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Huang, C.; Li, W. Oregano Essential Oil/β-cyclodextrin Inclusion Compound Polylactic Acid/Polycaprolactone Electrospun Nanofibers for Active Food Packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Herrera, N.; Olsén, P.; Berglund, L. Strongly Improved Mechanical Properties of Thermoplastic Biocomposites by PCL-Grafting inside Holocellulose Wood Fibers. ACS Sustain. Chem. Eng. 2020, 8, 11977–11985. [Google Scholar] [CrossRef]
- Yang, X.; Ku, T.H.; Biswas, S.K.; Yano, H.; Abe, K. UV Grafting: Surface Modification of Cellulose Nanofibers without the Use of Organic Solvents. Green Chem. 2019, 21, 4619–4624. [Google Scholar] [CrossRef]
- Kaldéus, T.; Telaretti Leggieri, M.R.; Cobo Sanchez, C.; Malmström, E. All-Aqueous Si-Arget Atrp from Cellulose Nanofibrils Using Hydrophilic and Hydrophobic Monomers. Biomacromolecules 2019, 20, 1937–1943. [Google Scholar] [CrossRef]
- Lander, S.; Erlandsson, J.; Vagin, M.; Gueskine, V.; Korhonen, L.; Berggren, M.; Wågberg, L.; Crispin, X. Sulfonated Cellulose Membranes: Physicochemical Properties and Ionic Transport Versus Degree of Sulfonation. Adv. Sustain. Syst. 2022, 6, 2200275. [Google Scholar] [CrossRef]
- Trovagunta, R.; Kelley, S.S.; Lavoine, N. Dual-Templating Approach for Engineering Strong, Biodegradable Lignin-Based Foams. ACS Sustain. Chem. Eng. 2022, 10, 15058–15067. [Google Scholar] [CrossRef]
- Rahimi Kord Sofla, M.; Batchelor, W.; Kosinkova, J.; Pepper, R.; Brown, R.; Rainey, T. Cellulose Nanofibres from Bagasse Using a High Speed Blender and Acetylation as a Pretreatment. Cellulose 2019, 26, 4799–4814. [Google Scholar] [CrossRef]
- Vega-Hernández, M.Á.; Cano-Díaz, G.S.; Vivaldo-Lima, E.; Rosas-Aburto, A.; Hernández-Luna, M.G.; Martinez, A.; Palacios-Alquisira, J.; Mohammadi, Y.; Penlidis, A. A Review on the Synthesis, Characterization, and Modeling of Polymer Grafting. Processes 2021, 9, 375. [Google Scholar] [CrossRef]
- binti Hashim, H.; binti Emran, N.A.; Isono, T.; Katsuhara, S.; Ninoyu, H.; Matsushima, T.; Yamamoto, T.; Borsali, R.; Satoh, T.; Tajima, K. Improving the Mechanical Properties of Polycaprolactone Using Functionalized Nanofibrillated Bacterial Cellulose with High Dispersibility and Long Fiber Length as a Reinforcement Material. Compos. Part A Appl. Sci. Manuf. 2022, 158, 106978. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Shen, Z.; Shu, X.; Sun, R. Preparation of Cellulose-Graft-Poly(ε-caprolactone) Nanomicelles by Homogeneous Rop in Ionic Liquid. Carbohydr. Polym. 2013, 921, 77–83. [Google Scholar] [CrossRef]
- Zuppolini, S.; Maya, I.C.; Diodato, L.; Guarino, V.; Borriello, A.; Ambrosio, L. Self-Associating Cellulose-Graft-Poly(ε-caprolactone) to Design Nanoparticles for Drug Release. Mater. Sci. Eng. C 2020, 108, 110385. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Gao, Q.; Yin, L.; Zhang, S. Synthesis and Catalytic Performance of Banana Cellulose Nanofibres Grafted with Poly(ε-caprolactone) in a Novel Two-Dimensional Zinc(II) Metal-Organic Framework. Int. J. Biol. Macromol. 2023, 224, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Goffin, A.L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites Based on Poly(ε-caprolactone)-Grafted Cellulose Nanocrystals by Ring-Opening Polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Li, K.; Song, J.; Xu, M.; Kuga, S.; Zhang, L.; Cai, J. Extraordinary Reinforcement Effect of Three-Dimensionally Nanoporous Cellulose Gels in Poly(ε-caprolactone) Bionanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 7204–7213. [Google Scholar] [CrossRef]
- Yu, C.; Cen, X.; Ao, D.; Qiao, Z.; Zhong, C. Preparation of Thin-Film Composite Membranes with Ultrahigh Mofs Loading through Polymer-Template Mofs Induction Secondary Interfacial Polymerization. Appl. Surf. Sci. 2023, 614, 156186. [Google Scholar] [CrossRef]
- Zhou, C.H.; Long, J.R.; Yaghi, M.O. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Wu, Y.P.; Tian, J.W.; Liu, S.; Li, B.; Zhao, J.; Ma, L.F.; Li, D.S.; Lan, Y.Q.; Bu, X. Bi-Microporous Metal–Organic Frameworks with Cubane [M4(OH)4] (M=Ni, Co) Clusters and Pore-Space Partition for Electrocatalytic Methanol Oxidation Reaction. Angew. Chem. Int. Ed. 2019, 58, 12185–12189. [Google Scholar] [CrossRef]
- Yu, C.X.; Wang, K.Z.; Li, X.J.; Liu, D.; Ma, L.F.; Liu, L.L. Highly Efficient and Facile Removal of Pb2+ from Water by Using a Negatively Charged Azoxy-Functionalized Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 5251–5260. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, X.; Qiu, J.; Gahungu, G.; Yuan, H.; Zhang, J. Water-Robust Zinc–Organic Framework with Mixed Nodes and Its Handy Mixed-Matrix Membrane for Highly Effective Luminescent Detection of Fe3+, CrO42−, and Cr2O72− in Aqueous Solution. Inorg. Chem. 2021, 60, 1716–1725. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.R.; Li, J.R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.C. Potential Applications of Metal-Organic Frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Haldar, R.; Bhattacharyya, S.; Maji, T.K. Luminescent Metal–Organic Frameworks and Their Potential Applications. J. Chem. Sci. 2020, 132, 99. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, Y.; Shi, X.; Lu, Z.; Ren, J.; Wang, Z.; Xu, J.; Fan, Y.; Wang, L. Eu-MOF and Its Mixed-Matrix Membranes as a Fluorescent Sensor for Quantitative Ratiometric PH and Folic Acid Detection, and Visible Fingerprint Identifying. Inorg. Chem. Front. 2021, 8, 4924–4932. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, P.; Jia, S.; Pan, H.; Zhang, H.; Wang, D.; Dong, L. Exploring Polylactide/Poly(Butylene Adipate-co-Terephthalate)/Rare Earth Complexes Biodegradable Light Conversion Agricultural Films. Int. J. Biol. Macromol. 2019, 127, 210–221. [Google Scholar] [CrossRef]
- Nouar, F.; Devic, T.; Chevreau, H.; Guillou, N.; Gibson, E.; Clet, G.; Daturi, M.; Vimont, A.; Grenèche, J.M.; Breeze, M.I.; et al. Tuning the Breathing Behaviour of MIL-53 by Cation Mixing. Chem. Commun. 2012, 48, 10237–10239. [Google Scholar] [CrossRef]
- Depauw, H.; Nevjestić, I.; De Winne, J.; Wang, G.; Haustraete, K.; Leus, K.; Verberckmoes, A.; Detavernier, C.; Callens, F.; De Canck, E.; et al. Microwave Induced “Egg Yolk” Structure in Cr/V-MIL-53. Chem. Commun. 2017, 53, 8478–8481. [Google Scholar] [CrossRef]
- Guo, P.; Fu, Q.; Yildiz, C.; Chen, Y.T.; Ollegott, K.; Froese, C.; Kleist, W.; Fischer, R.A.; Wang, Y.; Muhler, M.; et al. Regulating the Size and Spatial Distribution of Pd Nanoparticles Supported by the Defect Engineered Metal–Organic Framework HKUST-1 and Applied in the Aerobic Oxidation of Cinnamyl Alcohol. Catal. Sci. Technol. 2019, 9, 3703–3710. [Google Scholar] [CrossRef]
- Gao, W.; Wei, H.; Wang, C.L.; Liu, J.P.; Zhang, X.M. Multifunctional Zn–Ln (Ln = Eu and Tb) Heterometallic Metal–Organic Frameworks with Highly Efficient I2 Capture, Dye Adsorption, Luminescence Sensing and White-Light Emission. Dalton Trans. 2021, 50, 11619–11630. [Google Scholar] [CrossRef]
- Wang, L.J.; Deng, H.; Furukawa, H.; Gándara, F.; Cordova, K.E.; Peri, D.; Yaghi, O.M. Synthesis and Characterization of Metal–Organic Framework-74 Containing 2, 4, 6, 8, and 10 Different Metals. Inorg. Chem. 2014, 53, 5881–5883. [Google Scholar] [CrossRef]
- Saliba, D.; Ammar, M.; Rammal, M.; Al-Ghoul, M.; Hmadeh, M. Crystal Growth of ZIF-8, ZIF-67, and Their Mixed-Metal Derivatives. J. Am. Chem. Soc. 2018, 140, 1812–1823. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Li, H.; Chen, X. Syntheses, Structures and Luminescent Properties of Eu- and Tb-MOFs with 3,5-Pyridinedicarboxylate and 1,2-Benzenedicarboxylate. J. Fluoresc. 2021, 31, 1393–1399. [Google Scholar] [CrossRef]
- Gao, Q.F.; Jiang, T.L.; Li, W.Z.; Tan, D.F.; Zhang, X.H.; Pang, J.Y.; Zhang, S.H. Porous and Stable Zn-Series Metal–Organic Frameworks as Efficient Catalysts for Grafting Wood Nanofibers with Polycaprolactone via a Copolymerization Approach. Inorg. Chem. 2023, 62, 3464–3473. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Tan, D.; Huang, C.; Jiang, T.; Gao, Q.; Mo, X.; Zhang, S. Non-Isothermal Crystallization Kinetics of Polycaprolactone-Based Composite Membranes. J. Polym. Res. 2022, 29, 479. [Google Scholar] [CrossRef]
- Sheldrick, G. ShELXT@ Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Chen, X.; Chen, T.T.; Li, W.L.; Lu, J.B.; Zhao, L.J.; Jian, T.; Hu, H.S.; Wang, L.S.; Li, J. Lanthanides with Unusually Low Oxidation States in the PrB3− and PrB4− Boride Clusters. Inorg. Chem. 2018, 58, 411–418. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Majumder, A.; Rosair, G.M.; Mallick, A.; Chattopadhyay, N.; Mitra, S. Synthesis, Structures and Fluorescence of Nickel, Zinc and Cadmium Complexes with the N,N,O-Tridentate Schiff Base N-2-Pyridylmethylidene-2-Hydroxy-Phenylamine. Polyhedron 2006, 25, 1753–1762. [Google Scholar] [CrossRef]
- Yin, L.; Deng, Q.; Ke, Z.; Yu, Q.; Xiao, Y.; Zhang, S. Synthesis, Modification, and Adsorption Properties of Yb-MOF: Kinetic and Thermodynamic Studies. Appl. Organomet. Chem. 2022, 37, e6955. [Google Scholar] [CrossRef]
- Reglinski, J.; Morris, S.; Stevenson, D.E. Supporting Conformational Change at Metal Centres. Part 2: Four and Five Coordinate Geometry. Polyhedron 2002, 21, 2175–2182. [Google Scholar] [CrossRef]
- Nag, J.K.; Pal, S.; Sinha, C. Synthesis and Characterization of Cobalt(II), Nickel(II), Copper(II), Palladium(II) and Dioxouranium(VI) Complexes of the Antipyrine Schiff Base of 3-Formylsalicylic Acid. Transit. Met. Chem. 2005, 30, 523–526. [Google Scholar] [CrossRef]
- Patron, L.; Carp, O.; Mindru, I.; Marinescu, G.; Segal, E. Iron, Nickel and Zinc Malates Coordination Compounds Cynthesis, Characterization and Thermal Behaviour. J. Therm. Anal. Calorim. 2003, 72, 281–288. [Google Scholar] [CrossRef]
- Sattayanon, C.; Kungwan, N.; Punyodom, W. Theoretical Investigation on the Mechanism and Kinetics of the Ring-Opening Polymerization of ε-caprolactone Initiated by Tin(II) Alkoxides. J. Mol. Model. 2013, 19, 5377–5385. [Google Scholar] [CrossRef]
- Sattayanon, C.; Sontising, W.; Jitonnom, J.; Meepowpan, P.; Punyodom, W.; Kungwan, N. Theoretical Study on the Mechanism and Kinetics of Ring-Opening Polymerization of Cyclic Esters Initiated by Tin(II) N-Butoxide. Comput. Theor. Chem. 2014, 1044, 29–35. [Google Scholar] [CrossRef]
- Gümüta, S.; Balcan, M.; Knal, A. Computational Determination of Ring Opening Polymerization Reaction Mechanism of α-Angelica Lactone. Comput. Theor. Chem. 2018, 1142, 1–8. [Google Scholar] [CrossRef]
- Lyubov, D.M.; Tolpygin, A.O.; Trifonov, A.A. Rare-Earth Metal Complexes as Catalysts for Ring-Opening Polymerization of Cyclic Esters. Coord. Chem. Rev. 2019, 392, 83–145. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, C.; Wang, H.; Zhu, X.; Yang, G.; Wang, S. Synthesis, Characterization, and Catalytic Activities of Rare-Earth Metal Complexes with Iminopyrrolyl Ligands. Inorg. Chem. Commun. 2011, 14, 1196–1200. [Google Scholar] [CrossRef]
- Dai, X.; Shi, X.; Huo, C.; Wang, X. Study on the Poly(Lactic Acid)/Nano MOFs Composites: Insights into the MOFs-Induced Crystallization Mechanism and the Effects of MOFs on the Properties of the Composites. Thermochim. Acta 2017, 657, 39–46. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, Y.; Liu, H.; Ying, J.; Liu, C.; Shen, C. Effects of Interface Interaction and Microphase Dispersion on the Mechanical Properties of PCL/PLA/MMT Nanocomposites Visualized by Nanomechanical Mapping. Compos. Sci. Technol. 2020, 190, 108048. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Kim, M.S.; Hyun, H.; Seo, K.S.; Cho, Y.H.; Won Lee, J.; Rae Lee, C.; Khang, G.; Lee, H.B. Preparation and Characterization of MPEG–PCL Diblock Copolymers with Thermo-Responsive Sol–Gel–Sol Phase Transition. J. Polym. Sci. A Polym. Chem. 2006, 44, 5413–5423. [Google Scholar] [CrossRef]
- Trakoolwannachai, V.; Kheolamai, P.; Ummartyotin, S. Characterization of Hydroxyapatite from Eggshell Waste and Polycaprolactone (PCL) Composite for Scaffold Material. Compos. B Eng. 2019, 173, 106974. [Google Scholar] [CrossRef]
- Song, L.; Guo, Y.; Fan, J.; Fan, X.; Xie, Y.; Xiao, Z.; Wang, H.; Liang, D.; Wang, Y. Alkyl Thiol Grafted Silver Nanoparticle–Decorated Cellulose Nanocrystals on Poly(Lactic Acid) Composites for Enhanced Antibacterial Activity and Toughening Effects. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107231. [Google Scholar] [CrossRef]
- Lu, T.; Liu, S.; Jiang, M.; Xu, X.; Wang, Y.; Wang, Z.; Gou, J.; Hui, D.; Zhou, Z. Effects of Modifications of Bamboo Cellulose Fibers on the Improved Mechanical Properties of Cellulose Reinforced Poly(Lactic Acid) Composites. Compos. B Eng. 2014, 62, 191–197. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Uma Ravi Sankar, A.; Varalakshmi, M.; Kiran, Y.B.; Rambabu, G.; Yoon, K.R. Design and Synthesis of New Binuclear Photo Luminescent Europium (III) Complex. Inorg. Chem. Commun. 2022, 139, 109328. [Google Scholar] [CrossRef]
- Dandekar, M.P.; Itankar, S.G.; Kondawar, S.B.; Nandanwar, D.V.; Koinkar, P. Photoluminescent Electrospun Europium Complex Eu(TTA)3phen Embedded Polymer Blends Nanofibers. Opt. Mater. 2018, 85, 483–490. [Google Scholar] [CrossRef]
- Jiménez, G.L.; Rosales-Hoz, M.J.; Leyva, M.A.; Reyes-Rodríguez, J.L.; Galindo-García, U.; Falcony, C. Structural Analysis of an Europium-Sodium Complex Containing 2-Thenoyltrifluoroacetone and Succinimide as Ligands, a Highly Photoluminescent Material. J. Mol. Struct. 2021, 1228, 129778. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, X.; Han, T.; Wang, S.; Lin, Z.; Dong, Y.; Wang, B. Tuning the Luminescence of Metal–Organic Frameworks for Detection of Energetic Heterocyclic Compounds. J. Am. Chem. Soc. 2014, 136, 15485–15488. [Google Scholar] [CrossRef]
- Li, G.Y.; Zhao, G.J.; Liu, Y.H.; Han, K.L.; He, G.Z. TD-DFT Study on the Sensing Mechanism of a Fluorescent Chemosensor for Fluoride: Excited-State Proton Transfer. J. Comput. Chem. 2010, 31, 1759–1765. [Google Scholar] [CrossRef]







| Sample | [AGU]/[ε-CL] | Catalyst (wt%) | T (°C) | MSa | WPCL (%) b | Ref. |
|---|---|---|---|---|---|---|
| CGCL c | 1:10 | 2% Sn(Oct)2 | 130 | 0.68 | 32.36 | [20] |
| BGCL c | 1:50 | 2% Zn-MOF | 120 | 5.72 | 80.10 | [22] |
| GCL c | 1:10 | 2% Sn(Oct)2 | 120 | 1.32 | 48.07 | [41] |
| GCL c | 1:10 | 2% DMAP | 120 | 0.42 | 22.48 | [41] |
| GCL c | 1:10 | 2% UiO-67 | 120 | 1.45 | 50.41 | [41] |
| GCL2 | 1:10 | 2% MOF 2 | 120 | 1.09 | 43.52 | [41] |
| GCL1 | 1:10 | 2% MOF 1 | 120 | 4.38 | 75.52 | [41] |
| GCL1 | 1:30 | 2% MOF 1 | 120 | 9.56 | 87.05 | [41] |
| GCL | 1:30 | 2% ZnEu-MOF | 120 | 5.47 | 79.38 | This work |
| Sample | Tc (°C) | Tm (°C) | ΔHm (J/g) | ΔHc (J/g) | |
|---|---|---|---|---|---|
| PCL | 29.28 | 55.82 | 52.08 | 56.31 | 38.29% |
| 2 wt% LCF | 28.59 | 56.27 | 52.98 | 59.35 | 39.75% |
| 4 wt% LCF | 28.53 | 55.16 | 52.63 | 46.90 | 40.31% |
| 6 wt% LCF | 26.28 | 55.99 | 53.36 | 50.37 | 41.74% |
| 8 wt% LCF | 26.02 | 54.98 | 56.14 | 57.58 | 44.87% |
| 10 wt% LCF | 25.61 | 55.50 | 58.86 | 59.47 | 47.04% |
| Film Number | PCL | 2 wt% LCF | 4 wt% LCF | 6 wt% LCF | 8 wt% LCF | 10 wt% LCF |
|---|---|---|---|---|---|---|
| Excitation wavelength | - | 400.6 nm | 403.2 nm | 401.0 nm | 397.0 nm | 402.8 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, J.; Jiang, T.; Ke, Z.; Xiao, Y.; Li, W.; Zhang, S.; Guo, P. Wood Cellulose Nanofibers Grafted with Poly(ε-caprolactone) Catalyzed by ZnEu-MOF for Functionalization and Surface Modification of PCL Films. Nanomaterials 2023, 13, 1904. https://doi.org/10.3390/nano13131904
Pang J, Jiang T, Ke Z, Xiao Y, Li W, Zhang S, Guo P. Wood Cellulose Nanofibers Grafted with Poly(ε-caprolactone) Catalyzed by ZnEu-MOF for Functionalization and Surface Modification of PCL Films. Nanomaterials. 2023; 13(13):1904. https://doi.org/10.3390/nano13131904
Chicago/Turabian StylePang, Jinying, Tanlin Jiang, Zhilin Ke, Yu Xiao, Weizhou Li, Shuhua Zhang, and Penghu Guo. 2023. "Wood Cellulose Nanofibers Grafted with Poly(ε-caprolactone) Catalyzed by ZnEu-MOF for Functionalization and Surface Modification of PCL Films" Nanomaterials 13, no. 13: 1904. https://doi.org/10.3390/nano13131904