Lipase-Responsive Amphotericin B Loaded PCL Nanoparticles for Antifungal Therapies
Abstract
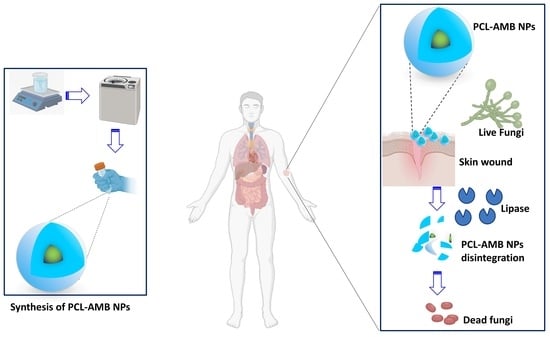
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AMB Loaded Nanoparticles (PCL-AMB NPs)
2.3. Physicochemical Characterization of Nanoparticles
2.4. Quantification of AMB by High-Performance Liquid Chromatography (HPLC)
2.5. Drug Loading and Encapsulation Efficiency
2.6. In Vitro Drug Release Assay
2.7. In Vitro Antifungal Activity of PCL-AMB NPs
2.7.1. Culturing of Candida albicans (C. albicans)
2.7.2. Minimum Inhibitory Concentration (MIC) Assay
2.7.3. Disk Diffusion Assay
2.8. Cytotoxicity Assay
2.9. Statistical Data Analysis
3. Results and Discussion
3.1. Nanoparticle Synthesis and Physicochemical Characterization
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Differential Scanning Calorimetry (DSC)
3.4. In Vitro Drug Release
3.5. Antifungal Efficacy of PCL-AMB NPs
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Caceres, D.H.; Tap, R.M.; Alastruey-Izquierdo, A.; Hagen, F. Detection and Control of Fungal Outbreaks. Mycopathologia 2020, 185, 741–745. [Google Scholar] [CrossRef]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microb. Cell 2020, 7, 143–145. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Genet. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Spampinato, C.; Leonardi, D. Candida Infections, Causes, Targets, and Resistance Mechanisms: Traditional and Alternative Antifungal Agents. BioMed Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [Green Version]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal Drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Yu, Y.; Peng, G.; Sun, M.; Lv, P.; Li, X. Amphotericin B and Curcumin Co-Loaded Porous Microparticles as a Sustained Release System against Candida albicans. Molecules 2022, 27, 3079. [Google Scholar] [CrossRef]
- Yang, M.; Xie, S.; Adhikari, V.P.; Dong, Y.; Du, Y.; Li, D. The synergistic fungicidal effect of low-frequency and low-intensity ultrasound with amphotericin B-loaded nanoparticles on C. albicans in vitro. Int. J. Pharm. 2018, 542, 232–241. [Google Scholar] [CrossRef]
- Bassi, R.C.; Boriollo, M.F. Amphotericin B, fluconazole, and nystatin as development inhibitors of Candida albicans biofilms on a dental prosthesis reline material: Analytical models in vitro. J. Prosthet. Dent. 2022, 127, 320–330. [Google Scholar] [CrossRef]
- Shizari, L.N.; Dounighi, N.M.; Bayat, M.; Mosavari, N. A New Amphotericin B-loaded Trimethyl Chitosan Nanoparticles as a Drug Delivery System and Antifungal Activity on Candida albicans Biofilm. Arch. Razi Inst. 2021, 76, 575–590. [Google Scholar] [CrossRef]
- Yang, D.; Lv, X.; Xue, L.; Yang, N.; Hu, Y.; Weng, L.; Fu, N.; Wang, L.; Dong, X. A lipase-responsive antifungal nanoplatform for synergistic photodynamic/photothermal/pharmaco-therapy of azole-resistant Candida albicans infections. Chem. Commun. 2019, 55, 15145–15148. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W.; et al. An NIR Discrete Metallacycle Constructed from Perylene Bisimide and Tetraphenylethylene Fluorophores for Imaging-Guided Cancer Radio-Chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef] [PubMed]
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4001330/ (accessed on 10 September 2022). [PubMed]
- Homaeigohar, S.; Boccaccini, A.R. Nature-Derived and Synthetic Additives to poly (ɛ-Caprolactone) Nanofibrous Systems for Biomedicine; an Updated Overview. Front. Chem. 2022, 9, 809676. [Google Scholar] [CrossRef]
- Saqib, M.; Ali Bhatti, A.S.; Ahmad, N.M.; Ahmed, N.; Shahnaz, G.; Lebaz, N.; Elaissari, A. Amphotericin B Loaded Polymeric Nanoparticles for Treatment of Leishmania Infections. Nanomaterials 2020, 10, 1152. [Google Scholar] [CrossRef]
- Kim, S.M.; Patel, M.; Patel, R. PLGA Core-Shell Nano/Microparticle Delivery System for Biomedical Application. Polymers 2021, 13, 3471. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, G.C.; Pandey, K.; Das, V.; Das, P. Study the effects of PLGA-PEG encapsulated Amphotericin B nanoparticle drug delivery system against Leishmania donovani. Drug Deliv. 2015, 22, 383–388. [Google Scholar] [CrossRef]
- Palma, E.; Pasqua, A.; Gagliardi, A.; Britti, D.; Fresta, M.; Cosco, D. Antileishmanial Activity of Amphotericin B-loaded-PLGA Nanoparticles: An Overview. Materials 2018, 11, 1167. [Google Scholar] [CrossRef] [Green Version]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Ansary, R.H.; Awang, M.B.; Rahman, M.M. Biodegradable Poly(D,L-lactic-co-glycolic acid)-Based Micro/Nanoparticles for Sustained Release of Protein Drugs—A Review. Trop. J. Pharm. Res. 2014, 13, 1179–1190. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Das, S.; De, A.K.; Kar, N.; Bera, T. Amphotericin B-loaded mannose modified poly(d,l-lactide-co-glycolide) polymeric nanoparticles for the treatment of visceral leishmaniasis: In vitro and in vivo approaches. RSC Adv. 2017, 7, 29575–29590. [Google Scholar] [CrossRef] [Green Version]
- Carraro, T.C.M.M.; Khalil, N.M.; Mainardes, R.M. Amphotericin B-loaded polymeric nanoparticles: Formulation optimization by factorial design. Pharm. Dev. Technol. 2016, 21, 140–146. [Google Scholar] [CrossRef]
- Stepanov, A.A.; Poshvina, D.V.; Vasilchenko, A.S. 2,4-Diacetylphloroglucinol Modulates Candida albicans Virulence. J. Fungi 2022, 8, 1018. [Google Scholar] [CrossRef] [PubMed]
- DiGiammarino, E.; McGrath, K. Detection of Secreted Lipase Proteins from Candida Species. US20070134747A1, 14 June 2007. Available online: https://patents.google.com/patent/US20070134747A1/en (accessed on 18 September 2022).
- Jothi, R.; Sangavi, R.; Kumar, P.; Pandian, S.K.; Gowrishankar, S. Author Correction: Catechol thwarts virulent dimorphism in Candida albicans and potentiates the antifungal efficacy of azoles and polyenes. Sci. Rep. 2021, 11, 22545. [Google Scholar] [CrossRef]
- Thewes, S.; Moran, G.P.; Magee, B.B.; Schaller, M.; Sullivan, D.J.; Hube, B. Phenotypic screening, transcriptional profiling, and comparative genomic analysis of an invasive and non-invasive strain of Candida albicans. BMC Microbiol. 2008, 8, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budzyńska, A.; Sadowska, B.; Więckowska-Szakiel, M.; Różalska, B. Enzymatic profile, adhesive and invasive properties of Candida albicans under the influence of selected plant essential oils. Acta Biochim. Pol. 2014, 61, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Bartlett, M.; Chaturvedi, V.; Ghannoum, M.; Hazen, K.; Pfaller, M.; Rinaldi, M.; Walsh, T. Optimal susceptibility testing conditions for detection of azole resistance in Aspergillus spp.: NCCLS collaborative evaluation. Antimicrob. Agents Chemother. 2001, 45, 1828–1835. [Google Scholar] [CrossRef] [Green Version]
- Marcano, R.G.D.J.V.; Tominaga, T.T.; Khalil, N.M.; Pedroso, L.S.; Mainardes, R.M. Chitosan functionalized poly (ε-caprolactone) nanoparticles for amphotericin B delivery. Carbohydr. Polym. 2018, 202, 345–354. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Mikołajczyk, A.; Błasiak, E.; Fic, E.; Dziedzicka-Wasylewska, M. Polycaprolactone Nanoparticles as Promising Candidates for Nanocarriers in Novel Nanomedicines. Pharmaceutics 2021, 13, 191. [Google Scholar] [CrossRef]
- Messeder, M.M.D.S.; Miranda, D.; de Souza, S.O.L.; Dorneles, M.; Giunchetti, R.; Oréfice, R.L. Positively-charged electrosprayed nanoparticles based on biodegradable polymers containing amphotericin B for the treatment of leishmaniasis. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 1189–1202. [Google Scholar] [CrossRef]
- Liverani, L.; Lacina, J.; Roether, J.A.; Boccardi, E.; Killian, M.S.; Schmuki, P.; Schubert, D.W.; Boccaccini, A.R. Incorporation of bioactive glass nanoparticles in electrospun PCL/chitosan fibers by using benign solvents. Bioact. Mater. 2018, 3, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Al-Quadeib, B.T.; Radwan, M.A.; Siller, L.; Horrocks, B.; Wright, M.C. Stealth Amphotericin B nanoparticles for oral drug delivery: In vitro optimization. Saudi Pharm. J. 2015, 23, 290–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehenni, L.; Lahiani-Skiba, M.; Ladam, G.; Hallouard, F.; Skiba, M. Preparation and Characterization of Spherical Amorphous Solid Dispersion with Amphotericin B. Pharmaceutics 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, S.; Kumar, V.; Sharma, K.; Nagpal, K.; Bera, T. Significance of Algal Polymer in Designing Amphotericin B Nanoparticles. Sci. World J. 2014, 2014, 564573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Göttel, B.; Lucas, H.; Syrowatka, F.; Knolle, W.; Kuntsche, J.; Heinzelmann, J.; Viestenz, A.; Mäder, K. In situ Gelling Amphotericin B Nanofibers: A New Option for the Treatment of Keratomycosis. Front. Bioeng. Biotechnol. 2020, 8, 600384. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Shin, B.-K.; Garripelli, V.K.; Kim, J.-K.; Davaa, E.; Jo, S.; Park, J.-S. A thermosensitive vaginal gel formulation with HPγCD for the pH-dependent release and solubilization of amphotericin B. Eur. J. Pharm. Sci. 2010, 41, 399–406. [Google Scholar] [CrossRef]
- McKeen, L.W. The effect of heat aging on the properties of sustainable polymers. In The Effect of Long Term Thermal Exposure on Plastics and Elastomers; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 313–332. [Google Scholar] [CrossRef]
- Li, R.; Song, Y.; Fouladian, P.; Arafat, M.; Chung, R.; Kohlhagen, J.; Garg, S. Three-dimensional printing of curcumin-loaded biodegradable and flexible scaffold for intracranial therapy of glioblastoma multiforme. Pharmaceutics 2021, 13, 471. [Google Scholar] [CrossRef]
- Arakawa, C.K.; DeForest, C.A. Polymer design and development. In Biology and Engineering of Stem Cell Niches; Elsevier: Amsterdam, The Netherlands, 2017; pp. 295–314. [Google Scholar]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Graninger, W.; Presterl, E. In vitro activity of antifungal combinations against Candida albicans biofilms. J. Antimicrob. Chemother. 2010, 65, 271–274. [Google Scholar] [CrossRef]
- al Jalali, V.; Sauermann, R.; Eberl, S.; Zeitlinger, M. In vitro activity of voriconazole and amphotericin B against Candida albicans, Candida krusei, and Cryptococcus neoformans in human cerebrospinal fluid. Infection 2019, 47, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, G.; Fathian-Kolahkaj, M.; Mohammadi, P.; Adibkia, K.; Fattahi, A. Preparation, physicochemical characterization and anti-fungal evaluation of amphotericin B-Loaded PLGA-PEG-galactosamine nanoparticles. Adv. Pharm. Bull. 2021, 11, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Genc, G.E.; Sahinkaya, O.; Demir, C.; Kisa, O.; Satana, D. In vitro antifungal activity of a medicinal plant extract mixture against candida species isolated from patients with oral stomatitis. Iran. Red Crescent Med. J. 2019, 21, e87251. [Google Scholar] [CrossRef]
- Zou, M.-L.; Teng, Y.-Y.; Wu, J.-J.; Liu, S.-Y.; Tang, X.-Y.; Jia, Y.; Chen, Z.-H.; Zhang, K.-W.; Sun, Z.-L.; Li, X.; et al. Fibroblasts: Heterogeneous cells with potential in regenerative therapy for scarless wound healing. Front. Cell Dev. Biol. 2021, 9, 713605. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]






| Sample | Hydrodynamic Diameter (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| PCL NPs | 198.9 ± 41.1 | 0.25 ± 0.04 | −21.5 ± 4.8 |
| PCL-AMB NPs | 393.5 ± 104.3 | 0.33 ± 0.1 | −7.0 ± 4.3 |
| Sample | Yield (%) | Drug Loading (%) | Encapsulation Efficiency (%) |
|---|---|---|---|
| PCL-AMB NPs | 46.9 ± 4.4 | 5.9 ± 0.5 | 42.0 ± 3.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uroro, E.O.; Bright, R.; Hayles, A.; Vasilev, K. Lipase-Responsive Amphotericin B Loaded PCL Nanoparticles for Antifungal Therapies. Nanomaterials 2023, 13, 155. https://doi.org/10.3390/nano13010155
Uroro EO, Bright R, Hayles A, Vasilev K. Lipase-Responsive Amphotericin B Loaded PCL Nanoparticles for Antifungal Therapies. Nanomaterials. 2023; 13(1):155. https://doi.org/10.3390/nano13010155
Chicago/Turabian StyleUroro, Evelyn Osehontue, Richard Bright, Andrew Hayles, and Krasimir Vasilev. 2023. "Lipase-Responsive Amphotericin B Loaded PCL Nanoparticles for Antifungal Therapies" Nanomaterials 13, no. 1: 155. https://doi.org/10.3390/nano13010155