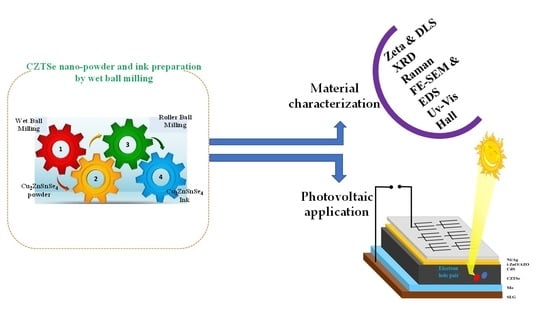
Synthesis and Characterization of Cu2ZnSnSe4 by Non-Vacuum Method for Photovoltaic Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Result and Discussion
3.1. Zeta Potential and DLS
3.2. Grazing-Incidence X-ray Diffraction (GI-XRD) Analysis
3.3. Raman Analysis
3.4. FT-IR Analysis
3.5. FE-SEM and EDS Analyses
3.6. Bulk XRD of Cu2ZnSnSe4 Thin Films
3.7. Optical Properties
3.8. Electrical Properties
3.9. Photovoltaic Analysis: J-V Characteristic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wibowo, R.A. Powder-to-film approach for fabricating critical raw material-free kesterite Cu2ZnSn (S,Se)4 thin film photovoltaic: A review. Sol. Energy 2018, 176, 157–169. [Google Scholar] [CrossRef]
- Luckert, F.; Hamilton, D.I.; Yakushev, M.V.; Beattie, N.S.; Zoppi, G.; Moynihan, M.; Forbes, I.; Karotki, A.V.; Mudryi, A.V.; Grossberg, M.; et al. Optical properties of high quality Cu2ZnSnSe4 thin films. Appl. Phys. Lett. 2011, 99, 062104. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Gershon, T.; Gunawan, O.; Todorov, T.K.; Gokmen, T.; Virgus, Y.; Guha, S. Cu2ZnSnSe4 thin-film solar cells by thermal co-evaporation with 11.6% efficiency and improved minority carrier diffusion length. Adv. Energy Mater. 2015, 5, 1401372. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, D.; Zhang, N.; Zhao, M.; Yu, X.; Liu, P.; Wei, Y.; Ren, G. Achieving 11.95% efficient Cu2ZnSnSe4 solar cells fabricated by sputtering a Cu–Zn–Sn–Se quaternary compound target with a selenization process. J. Mater. Chem. A 2019, 7, 9948–9957. [Google Scholar] [CrossRef]
- Brammertz, G.; Buffière, M.; Oueslati, S.; ElAnzeery, H.; Ben Messaoud, K.; Sahayaraj, S.; Köble, C.; Meuris, M.; Poortmans, J. Characterization of defects in 9.7% efficient Cu2ZnSnSe4-CdS-ZnO solar cells. Appl. Phys. Lett. 2013, 103, 163904. [Google Scholar] [CrossRef]
- Bag, S.; Gunawan, O.; Gokmen, T.; Zhu, Y.; Todorov, T.K.; Mitzi, D.B. Low band gap liquid-processed CZTSe solar cell with 10.1% efficiency. Energy Environ. Sci. 2012, 5, 7060–7065. [Google Scholar] [CrossRef]
- Jeon, J.O.; Lee, K.D.; Seul Oh, L.; Seo, S.W.; Lee, D.K.; Kim, H.; Jeong, J.H.; Ko, M.J.; Kim, B.; Son, H.J. Highly efficient copper–zinc–tin–selenide (CZTSe) solar cells by electrodeposition. ChemSusChem 2014, 7, 1073–1077. [Google Scholar] [CrossRef]
- Tiwari, K.J.; Ren, M.-Q.; Vajandar, S.K.; Osipowicz, T.; Subrahmanyam, A.; Malar, P. Mechanochemical bulk synthesis and e-beam growth of thin films of Sb2Se3 photovoltaic absorber. Sol. Energy 2018, 160, 56–63. [Google Scholar] [CrossRef]
- Tiwari, K.J.; Kumar, D.P.; Mallik, R.C.; Malar, P. Ball mill synthesis of bulk quaternary Cu2ZnSnSe4 and thermoelectric studies. J. Electron. Mater. 2017, 46, 30–39. [Google Scholar] [CrossRef]
- Rohini, M.; Reyes, P.; Velumani, S.; Latha, M.; Becerril-Juarez, I.; Asomoza, R. Parametric optimization of mechanochemical process for synthesis of Cu (In, Ga)0.5Se2 nanoparticles. Mater. Sci. Semicond. Process. 2015, 37, 151–158. [Google Scholar] [CrossRef]
- Goyal, D.; Goyal, C.; Ikeda, H.; Malar, P. Role of growth temperature in photovoltaic absorber CuSbSe2 deposition through e-beam evaporation. Mater. Sci. Semicond. Process. 2020, 108, 104874. [Google Scholar] [CrossRef]
- Shyju, T.; Anandhi, S.; Suriakarthick, R.; Gopalakrishnan, R.; Kuppusami, P. Mechanosynthesis, deposition and characterization of CZTS and CZTSe materials for solar cell applications. J. Solid State Chem. 2015, 227, 165–177. [Google Scholar] [CrossRef]
- Tiwari, K.J.; Mallik, R.C.; Malar, P. Raman studies of Ball mill synthesized bulk Cu2ZnSnSe4. In AIP Conference Proceedings; AIP Publishing LLC: Bhubaneswar, Odisha, India, 2017; Volume 1832, p. 060019. [Google Scholar]
- Goyal, D.; Malar, P. Dry mill route for synthesis of single phase bulk and e-beam growth of thin films of Cu2ZnSnSe4 for photovoltaic applications. J. Alloy. Compd. 2020, 846, 156464. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Liu, K.; Liu, H. Synthesis of Cu2ZnSnSe4 thin-film solar cells from nanoparticles by a non-vacuum mechanical ball milling and rapid thermal processing. Micro Nano Lett. 2020, 15, 887–891. [Google Scholar] [CrossRef]
- Tampo, H.; Kim, S.; Nagai, T.; Shibata, H. Sodium incorporation effect on morphological and photovoltaic properties for Cu2ZnSnSe4 solar cells. Jpn. J. Appl. Phys. 2020, 59, SCCD06. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, G.Y.; Nguyen, T.T.T.; Yoon, S.; Kim, Y.K.; Lee, S.Y.; Kim, M.; Cho, D.H.; Chung, Y.D.; Lee, J.H.; et al. Sodium-assisted passivation of grain boundaries and defects in Cu2ZnSnSe4 thin films. Phys. Chem. Chem. Phys. 2020, 22, 7597–7605. [Google Scholar] [CrossRef]
- Rehan, M.; Cho, A.; Amare, A.M.; Kim, K.; Yun, J.H.; Cho, J.S.; Park, J.H.; Gwak, J.; Shin, D. Defect passivation in Cu2ZnSnSe4 thin-film solar cells by novel sodium doping. Sol. Energy Mater Sol. Cells 2021, 228, 111138. [Google Scholar] [CrossRef]
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of heavy alkali elements in Cu (In, Ga)Se2 solar cells with efficiencies up to 22.6%. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Liu, W.; Zhang, Y.; Cheng, S.; Fan, Y.; Zhou, Z.; He, Q.; Zhang, Y.; Sun, Y. Adjustment of alkali element incorporations in Cu (In, Ga) Se2 thin films with wet chemistry Mo oxide as a hosting reservoir. Sol. Energy Mater Sol. Cells 2018, 174, 16–24. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, J.H.; Kim, W.M.; Park, J.K.; Baik, Y.J.; Seong, T.Y.; Jeong, J.h. Electrical properties of CIGS/Mo junctions as a function of MoSe2 orientation and Na doping. Prog. Photovolt. Res. Appl. 2014, 22, 90–96. [Google Scholar] [CrossRef]
- Hsu, C.H.; Su, Y.S.; Wei, S.Y.; Chen, C.H.; Ho, W.H.; Chang, C.; Wu, Y.H.; Lin, C.J.; Lai, C.H. Na-induced efficiency boost for Se-deficient Cu (In, Ga) Se2 solar cells. Prog. Photovolt.: Res. Appl. 2015, 23, 1621–1629. [Google Scholar] [CrossRef]
- Sahu, M.; Reddy, V.R.M.; Kim, B.; Patro, B.; Park, C.; Kim, W.K.; Sharma, P. Fabrication of Cu2ZnSnS4 Light Absorber Using a Cost-Effective Mechanochemical Method for Photovoltaic Applications. Materials 2022, 15, 1708. [Google Scholar] [CrossRef] [PubMed]
- Kadlag, K.P.; Rao, M.J.; Nag, A. Ligand-free, colloidal, and luminescent metal sulfide nanocrystals. J. Phys. Chem. Lett. 2013, 4, 1676–1681. [Google Scholar] [CrossRef]
- Huang, T.J.; Yin, X.; Tang, C.; Qi, G.; Gong, H. A low-cost, ligand exchange-free strategy to synthesize large-grained Cu2ZnSnS4 thin-films without a fine-grain underlayer from nanocrystals. J. Mater. Chem. A 2015, 3, 17788–17796. [Google Scholar] [CrossRef]
- Nag, A.; Kovalenko, M.V.; Lee, J.-S.; Liu, W.; Spokoyny, B.; Talapin, D.V. Metal-free inorganic ligands for colloidal nanocrystals: S2–, HS–, Se2–, HSe–, Te2–, HTe–, TeS32–, OH–, and NH2– as surface ligands. J. Am. Chem. Soc. 2011, 133, 10612–10620. [Google Scholar] [CrossRef] [PubMed]
- Prajapat, R.; Sharma, Y. Morphological characterization and microstructural study of Cu2ZnSnSe4 thin films with compositional variation. Mater. Res. Express 2019, 6, 116459. [Google Scholar] [CrossRef]
- Islam, M.; Rahman, K.; Haque, F.; Akhtaruzzaman, M.; Alam, M.; Alothman, Z.; Sopian, K.; Amin, N. Properties of low temperature vacuum annealed CZTS thin films deposited on polymer substrate. Chalcogenide Lett. 2014, 11, 233–239. [Google Scholar]
- Singh, A.K.; Aggarwal, G.; Singh, R.K.; Klein, T.R.; Das, C.; Neergat, M.; Kavaipatti, B.; van Hest, M.F.M. Synthesis of CZTS/Se and Their Solid Solution from Electrodeposited Cu–Sn–Zn Metal Precursor: A Study of S and Se Replacement Reaction. ACS Appl. Energy Mater 2018, 1, 3351–3358. [Google Scholar] [CrossRef]
- Shin, B.; Zhu, Y.; Bojarczuk, N.A.; Jay Chey, S.; Guha, S. Control of an interfacial MoSe2 layer in Cu2ZnSnSe4 thin film solar cells: 8.9% power conversion efficiency with a TiN diffusion barrier. Appl. Phys. Lett. 2012, 101, 053903. [Google Scholar] [CrossRef]
- Pareek, D.; Balasubramaniam, K.; Sharma, P. Synthesis and characterization of bulk Cu2ZnSnX4 (X: S, Se) via thermodynamically supported mechano-chemical process. Mater. Charact. 2015, 103, 42–49. [Google Scholar] [CrossRef]
- Amiri, N.B.M.; Postnikov, A. Electronic structure and lattice dynamics in kesterite-type Cu2ZnSnSe4 from first-principles calculations. Phys. Rev. B 2010, 82, 205204. [Google Scholar] [CrossRef]
- Wang, W.; Shen, H.; Wong, L.H.; Su, Z.; Yao, H.; Li, Y. A 4.92% efficiency Cu2ZnSnS4 solar cell from nanoparticle ink and molecular solution. RSC Adv. 2016, 6, 54049–54053. [Google Scholar] [CrossRef]
- Rehan, M.; Jeon, H.; Cho, Y.; Cho, A.; Kim, K.; Cho, J.-S.; Yun, J.H.; Ahn, S.; Gwak, J.; Shin, D. Fabrication and characterization of Cu2ZnSnSe4 thin-film solar cells using a single-stage Co-evaporation method: Effects of film growth temperatures on device performances. Energies 2020, 13, 1316. [Google Scholar] [CrossRef] [Green Version]
- Molla, A.; Sahu, M.; Kumar, Y.; Hussain, S. Soft template mediated synthesis of Bi–In–Zn–S and its efficient visible-light-driven decomposition of methylene blue. RSC Adv. 2015, 5, 41941–41948. [Google Scholar] [CrossRef] [Green Version]
- Molla, A.; Sahu, M.; Hussain, S. Under dark and visible light: Fast degradation of methylene blue in the presence of Ag–In–Ni–S nanocomposites. J. Mater. Chem. A 2015, 3, 15616–15625. [Google Scholar] [CrossRef] [Green Version]
- Marcos, M.A.; Cabaleiro, D.; Guimarey, M.J.; Comuñas, M.J.; Fedele, L.; Fernández, J.; Lugo, L. PEG 400-based phase change materials nano-enhanced with functionalized graphene nanoplatelets. Nanomaterials 2018, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gu, J.; Zhang, J.; Yu, F.; Wang, J.; Nie, N.; Li, W. LiFePO4 nanoparticles growth with preferential (010) face modulated by Tween-80. Rsc Adv. 2015, 5, 9745–9751. [Google Scholar] [CrossRef]
- Indubala, E.; Sarveshvaran, S.; Sudha, V.; Mamajiwala, A.Y.; Harinipriya, S. Secondary phases and temperature effect on the synthesis and sulfurization of CZTS. Sol. Energy 2018, 173, 215–224. [Google Scholar] [CrossRef]
- Kishore, R.S.; Pappenberger, A.; Dauphin, I.B.; Ross, A.; Buergi, B.; Staempfli, A.; Mahler, H.-C. Degradation of polysorbates 20 and 80: Studies on thermal autoxidation and hydrolysis. J. Pharm. Sci. 2011, 100, 721–731. [Google Scholar] [CrossRef]
- Pramono, E.; Utomo, S.; Wulandari, V.; Clegg, F. The effect of polyethylene glycol Mw 400 and 600 on stability of Shellac Waxfree. In J. Phys. Conf. Ser. IOP Publ. 2016, 7, 012054. [Google Scholar]
- Tang, D.; Wang, Q.; Liu, F.; Zhao, L.; Han, Z.; Sun, K.; Lai, Y.; Li, J.; Liu, Y. An alternative route towards low-cost Cu2ZnSnS4 thin film solar cells. Surf. Coat. Technol. 2013, 232, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, F.; Yang, B.; Peng, X.; Chen, Q.; Zou, J.; Dou, X. Fabrication of Cu2ZnSnS4 thin films based on facile nanocrystals-printing approach with rapid thermal annealing (RTA) process. Coatings 2019, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Kaupmees, L.; Altosaar, M.; Volobujeva, O.; Raadik, T.; Grossberg, M.; Danilson, M.; Mellikov, E.; Barvinschi, P. Isothermal and two-temperature zone selenization of Mo layers. Adv. Mater. Sci. Eng. 2012, 2012, 345762. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, G.; Xue, C.; Jin, X.; Liu, W.; Zhu, C. Cu2ZnSnS4− xSex solar cells fabricated with precursor stacked layer ZnS/Cu/SnS by a CBD method. Sol. Energy Mater Sol. Cells 2015, 137, 131–137. [Google Scholar] [CrossRef]
- Amal, M.I.; Kim, K.H. Crystallization of kesterite Cu2ZnSnS4 prepared by the sulfurization of sputtered Cu–Zn–Sn precursors. Thin Solid Film. 2013, 534, 144–148. [Google Scholar] [CrossRef]
- Jirage, S.; Pagare, P.; Garadkar, K.; Bhuse, V. Structural and morphological studies of chemically deposited nanocrystalline and nano-flower like Cu2ZnSnSe4 thin films. Mater. Today Proc. 2021, 45, 3950–3954. [Google Scholar] [CrossRef]
- Olgar, M.A.; Başol, B.; Atasoy, Y.; Tomakin, M.; Aygun, G.; Ozyuzer, L.; Bacaksız, E. Effect of heat treating metallic constituents on the properties of Cu2ZnSnSe4 thin films formed by a two-stage process. Thin Solid Film. 2017, 624, 167–174. [Google Scholar] [CrossRef]
- Ito, K.; Nakazawa, T. Electrical and optical properties of stannite-type quaternary semiconductor thin films. Jpn. J. Appl. Phys. 1988, 27, 2094. [Google Scholar] [CrossRef]
- Saragih, A.D.; Wubet, W.; Abdullah, H.; Abay, A.K.; Kuo, D.-H. Characterization of Ag-doped Cu2ZnSnSe4 bulks material and their application as thin film semiconductor in solar cells. Mater. Sci. Eng. B 2017, 225, 45–53. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Y.; Chen, Y.; Wang, B.; Wang, Y.; Zhou, J.; Liang, Z. Hot-injection synthesis of Cu-doped Cu2ZnSnSe4 nanocrystals to reach thermoelectric zT of 0.70 at 450 C. ACS Appl. Mater. Interfaces 2015, 7, 24403–24408. [Google Scholar] [CrossRef]
- Khadka, D.B.; Kim, S.; Kim, J. Ge-alloyed CZTSe thin film solar cell using molecular precursor adopting spray pyrolysis approach. RSC Adv. 2016, 6, 37621–37627. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Huang, J.; Liang, G.; Zhang, Y.; Rey, G.; Guo, F.; Su, Z.; Zhu, H.; Cai, L. Defect Control for 12.5% Efficiency Cu2ZnSnSe4 Kesterite Thin-Film Solar Cells by Engineering of Local Chemical Environment. Adv. Mater. 2020, 32, 2005268. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Gunawan, O.; Gokmen, T.; Zhu, Y.; Mitzi, D.B. Hydrazine-processed Ge-substituted CZTSe solar cells. Chem. Mater. 2012, 24, 4588–4593. [Google Scholar] [CrossRef]
- Gu, E.; Yan, C.; Liu, F.; Liu, Y.; Su, Z.; Zhang, K.; Chen, Z.; Li, J.; Liu, Y. Cu2ZnSnS4 thin film solar cells from coated nanocrystals ink. J. Mater. Sci. Mater. 2015, 26, 1932–1939. [Google Scholar] [CrossRef]
- Agarwal, M.; Patel, P.; Vijayan, O. Electrical studies on (Mo/W)Se2 single crystals. I. Electrical resistivity. Phys. Status Solidi 1983, 78, 133–136. [Google Scholar] [CrossRef]
- Sahu, M.; Reddy, V.R.M.; Park, C.; Sharma, P. Review article on the lattice defect and interface loss mechanisms in kesterite materials and their impact on solar cell performance. Sol. Energy 2021, 230, 13–58. [Google Scholar] [CrossRef]
- Shi, C.; Shi, G.; Chen, Z.; Yang, P.; Yao, M. Deposition of Cu2ZnSnS4 thin films by vacuum thermal evaporation from single quaternary compound source. Mater. Lett. 2012, 73, 89–91. [Google Scholar] [CrossRef]
- Altosaar, M.; Raudoja, J.; Timmo, K.; Danilson, M.; Grossberg, M.; Krustok, J.; Mellikov, E. Cu2Zn1–xCdxSn(Se1–y Sy)4 solid solutions as absorber materials for solar cells. Phys. Status Solidi (A) 2008, 205, 167–170. [Google Scholar] [CrossRef]
- Redinger, A.; Hönes, K.; Fontané, X.; Izquierdo-Roca, V.; Saucedo, E.; Valle, N.; Pérez-Rodríguez, A.; Siebentritt, S. Detection of a ZnSe secondary phase in coevaporated Cu2ZnSnSe4 thin films. Appl. Phys.Lett. 2011, 98, 101907. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, I.-H. High pressure Raman scattering of a co-evaporated Cu2SnSe3 thin film. Thin SolidFilm. 2018, 647, 9–12. [Google Scholar] [CrossRef]
- Chihi, A.; Bessais, B. Synthesis and characterization of Cu2SnSe3 thin films by electrodeposition route. Superlattices Microstruct. 2016, 97, 287–297. [Google Scholar] [CrossRef]











| Sl No. | Sample Name | Cu/Zn + Sn | Zn/Sn | Se/(Cu + Zn + Sn) |
|---|---|---|---|---|
| 1 | Se0 | 1.05 | 1.07 | 0.86 |
| 2 | Se1 | 0.95 | 1.19 | 0.85 |
| 3 | Se2 | 1.07 | 1.02 | 0.89 |
| 4 | Se3 | 0.91 | 1.32 | 0.92 |
| 5 | Se0_Na | 1.12 | 0.82 | 1.03 |
| 6 | Se1_Na | 1.22 | 0.83 | 0.89 |
| 7 | Se2_Na | 1.17 | 0.78 | 0.92 |
| 8 | Se3_Na | 1.12 | 1.05 | 0.98 |
| Sl. No. | Sample Name | Carrier Conc. (cm−3) | Mobility cm2/V·s | Resistivity (Ω cm) |
|---|---|---|---|---|
| 1 | Se0 | 2.10 × 1018 | 1.21 | 2.47 |
| 2 | Se1 | 1.07 × 1018 | 1.33 | 4.41 |
| 3 | Se2 | 1.05 × 1018 | 1.54 | 3.87 |
| 4 | Se3 | 1.80 × 1018 | 1.13 | 3.06 |
| 5 | Se0_Na | 1.13 × 1018 | 1.12 | 4.96 |
| 6 | Se1_Na | 2.04 × 1017 | 6.71 | 4.56 |
| 7 | Se2_Na | 6.14 × 1017 | 1.76 | 5.77 |
| 8 | Se3_Na | 1.61 × 1019 | 3.86 | 0.101 |
| Sl No. | Sample Name | Voc (mV) | Jsc (mA/cm2) | FF (%) | η (%) |
|---|---|---|---|---|---|
| 1 | SC-Se1 | 292 | 1.37 | 36.56 | 0.14 |
| 2 | SC-Se2 | 266 | 1.62 | 37.79 | 0.16 |
| 3 | SC-Se3 | 293 | 0.85 | 41.43 | 0.10 |
| 4 | SC-Se1_Na | 335 | 0.86 | 44.19 | 0.12 |
| 5 | SC-Se2_Na | 300 | 0.76 | 41.32 | 0.10 |
| 6 | SC-Se3_Na | 182 | 1.60 | 33.55 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahu, M.; Minnam Reddy, V.R.; Patro, B.; Park, C.; Kim, W.K.; Sharma, P. Synthesis and Characterization of Cu2ZnSnSe4 by Non-Vacuum Method for Photovoltaic Applications. Nanomaterials 2022, 12, 1503. https://doi.org/10.3390/nano12091503
Sahu M, Minnam Reddy VR, Patro B, Park C, Kim WK, Sharma P. Synthesis and Characterization of Cu2ZnSnSe4 by Non-Vacuum Method for Photovoltaic Applications. Nanomaterials. 2022; 12(9):1503. https://doi.org/10.3390/nano12091503
Chicago/Turabian StyleSahu, Meenakshi, Vasudeva Reddy Minnam Reddy, Bharati Patro, Chinho Park, Woo Kyoung Kim, and Pratibha Sharma. 2022. "Synthesis and Characterization of Cu2ZnSnSe4 by Non-Vacuum Method for Photovoltaic Applications" Nanomaterials 12, no. 9: 1503. https://doi.org/10.3390/nano12091503