Adsorption of Chromate Ions by Layered Double Hydroxide–Bentonite Nanocomposite for Groundwater Remediation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of MgAl-Cl Layered Double Hydroxide
2.3. Preparation of LDH–BT Nanocomposites
2.3.1. LDH–BT by Co-Precipitation (LDH–BT_CP)
2.3.2. LDH–BT by Exfoliation–Reassembly (LDH–BT_ER)
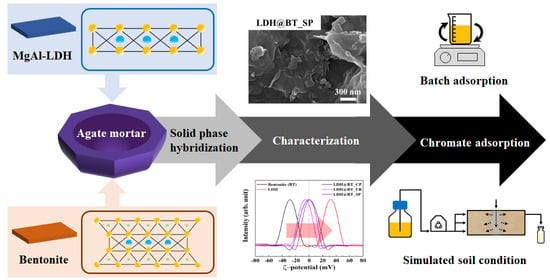
2.3.3. LDH–BT by Solid-Phase Hybridization (LDH–BT_SP)
2.4. Characterization
2.5. Chromate Adsorption Experiments
2.6. Mobility Test
2.7. Box Test
3. Results and Discussion
3.1. Characterization of the Parent Bentonite, the LDH, and the Various LDH–BT Nanocomposites
3.2. Chromate Adsorption Experiments
3.3. Mobility Test
3.4. Chromate Adsorption under Simulated Sub-Surface Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Losi, M.E.; Frankenberger, W.T. Chromium-resistant microorganisms isolated from evaporation ponds of a metal processing plant. Water Air Soil Pollut. 1994, 74, 405–413. [Google Scholar] [CrossRef]
- Camargo, F.A.O.; Bento, F.M.; Okeke, B.C.; Frankenberger, W.T. Chromate Reduction by Chromium-Resistant Bacteria Isolated from Soils Contaminated with Dichromate. J. Environ. Qual. 2003, 32, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Baruthio, F. Toxic effects of chromium and its compounds. Biol. Trace Elem. Res. 1992, 32, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef]
- Hong-wei, L. Adsorption behavior of soil and its somposing component to chromium. J. Dalian Natl. Univ. 2008, 5, 400–403. [Google Scholar]
- Hummel, M.; Standl, E.; Schnell, O. Chromium in metabolic and cardiovascular disease. Horm. Metab. Res. 2007, 39, 743–751. [Google Scholar] [CrossRef]
- Dattilo, A.M.; Miguel, S.G. Chromium in health and disease. Nutr. Today 2003, 38, 121–133. [Google Scholar] [CrossRef]
- Ramsey, J.D.; Xia, L.; Kendig, M.W.; McCreery, R.L. Raman spectroscopic analysis of the speciation of dilute chromate solutions. Corros. Sci. 2001, 43, 1557–1572. [Google Scholar] [CrossRef]
- WHO Organization; Staff, W.H.O. Guidelines for Drinking-Water Quality; IWA Publishing: London, UK, 2004. [Google Scholar]
- Katz, S.A.; Salem, H. The Biological and Environmental Chemistry of Chromium; VCH Publishers: Berlin, Germany, 1994. [Google Scholar]
- Castro-Rodríguez, A.; Carro-Pérez, M.E.; Iturbe-Argüelles, R.; González-Chávez, J.L. Adsorption of hexavalent chromium in an industrial site contaminated with chromium in Mexico. Environ. Earth Sci. 2015, 73, 175–183. [Google Scholar] [CrossRef]
- Bade, R.; Lee, S.H.; Jo, S.; Lee, H.-S.; Lee, S.-E. Micellar enhanced ultrafiltration (MEUF) and activated carbon fibre (ACF) hybrid processes for chromate removal from wastewater. Desalination 2008, 229, 264–278. [Google Scholar] [CrossRef]
- Laskaridis, A.; Sarakatsianos, I.; Tzollas, N.; Katsoyiannis, I.A. Simultaneous removal of arsenate and chromate from ground- and surface- waters by iron-based redox assisted coagulation. Sustainability 2020, 12, 5394. [Google Scholar] [CrossRef]
- Liu, T.; Rao, P.; Mak, M.S.H.; Wang, P.; Lo, I.M.C. Removal of co-present chromate and arsenate by zero-valent iron in groundwater with humic acid and bicarbonate. Water Res. 2009, 43, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Morita, M.; Matsuoka, M.; Tokoro, C. Sorption mechanisms of chromate with coprecipitated ferrihydrite in aqueous solution. J. Hazard. Mater. 2017, 334, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Chen, X.; Wang, D. Electrically regenerated ion exchange for removal and recovery of Cr(VI) from wastewater. Environ. Sci. Technol. 2007, 41, 1439–1443. [Google Scholar] [CrossRef]
- Yoon, J.; Amy, G.; Chung, J.; Sohn, J.; Yoon, Y. Removal of toxic ions (chromate, arsenate, and perchlorate) using reverse osmosis, nanofiltration, and ultrafiltration membranes. Chemosphere 2009, 77, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Alowitz, M.J.; Scherer, M.M. Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal. Environ. Sci. Technol. 2002, 36, 299–306. [Google Scholar] [CrossRef]
- Selvi, K.; Pattabhi, S.; Kadirvelu, K. Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour. Technol. 2001, 80, 87–89. [Google Scholar] [CrossRef]
- Wang, G.; Hua, Y.; Su, X.; Komarneni, S.; Ma, S.; Wang, Y. Cr(VI) adsorption by montmorillonite nanocomposites. Appl. Clay Sci. 2016, 124–125, 111–118. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Chávez, M.L.; de Pablo, L.; García, T.A. Adsorption of Ba2+ by Ca-exchange clinoptilolite tuff and montmorillonite clay. J. Hazard. Mater. 2010, 175, 216–223. [Google Scholar] [CrossRef]
- Huang, D.; Liu, C.; Zhang, C.; Deng, R.; Wang, R.; Xue, W.; Luo, H.; Zeng, G.; Zhang, Q.; Guo, X. Cr(VI) removal from aqueous solution using biochar modified with Mg/Al-layered double hydroxide intercalated with ethylenediaminetetraacetic acid. Bioresour. Technol. 2019, 276, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-J.; Choi, K.; Lee, S.; Cho, J.-M.; Choi, H.-J.; Hong, S.W.; Choi, J.-W.; Mizuseki, H.; Lee, W.-S. Chromate adsorption mechanism on nanodiamond-derived onion-like carbon. J. Hazard. Mater. 2016, 320, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Luo, H.; Chen, H.; Dong, T. Adsorption of chromate and para-nitrochlorobenzene on inorganic–organic montmorillonite. Appl. Clay Sci. 2011, 51, 198–201. [Google Scholar] [CrossRef]
- Barquist, K.; Larsen, S.C. Chromate adsorption on bifunctional, magnetic zeolite composites. Microporous Mesoporous Mat. 2010, 130, 197–202. [Google Scholar] [CrossRef]
- Sarkar, B.; Xi, Y.; Megharaj, M.; Krishnamurti, G.S.R.; Rajarathnam, D.; Naidu, R. Remediation of hexavalent chromium through adsorption by bentonite based Arquad® 2HT-75 organoclays. J. Hazard. Mater. 2010, 183, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.S.; Shannon, E.V. The minerals of bentonite and related clays and their physical properties1. J. Am. Ceram. Soc. 1926, 9, 77–96. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Ramachandran, M. Adsorptive removal of basic dyes from aqueous solutions by surfactant modified bentonite clay (organoclay): Kinetic and competitive adsorption isotherm. Process Saf. Environ. Protect. 2015, 95, 215–225. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Xu, Y. Chitosan and surfactant co-modified montmorillonite: A multifunctional adsorbent for contaminant removal. Appl. Clay Sci. 2017, 146, 35–42. [Google Scholar] [CrossRef]
- Duan, X.; Evans, D.G. Layered Double Hydroxides; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; Volume 119. [Google Scholar]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Chao, H.-P.; Wang, Y.-C.; Tran, H.N. Removal of hexavalent chromium from groundwater by Mg/Al-layered double hydroxides using characteristics of in-situ synthesis. Environ. Pollut. 2018, 243, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Khitous, M.; Salem, Z.; Halliche, D. Effect of interlayer anions on chromium removal using Mg–Al layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. Chin. J. Chem. Eng. 2016, 24, 433–445. [Google Scholar] [CrossRef]
- Li, Y.; Gao, B.; Wu, T.; Sun, D.; Li, X.; Wang, B.; Lu, F. Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide. Water Res. 2009, 43, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Ayuso, E.; Nugteren, H.W. Purification of chromium(VI) finishing wastewaters using calcined and uncalcined Mg-Al-CO3-hydrotalcite. Water Res. 2005, 39, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Kim, T.-H.; Bendix, J.; Abdelmoula, M.; Ruby, C.; Nielsen, U.G.; Bruun Hansen, H.C. Stability of magnetic LDH composites used for phosphate recovery. J. Colloid Interface Sci. 2020, 580, 660–668. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.; Zanta, C.L.P.S.; Soletti, J.I.; Ribeiro, L.M.O.; Dornelas, C.B.; Silva, T.L.; Vieira, M.G.A. MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl. Clay Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Y.; Liu, Q.; Wang, J.; Jing, X.; Liu, L.; Liu, J.; Song, D. Enhanced adsorption of uranium (VI) using a three-dimensional layered double hydroxide/graphene hybrid material. Chem. Eng. J. 2015, 259, 752–760. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Kazeem, T.S.; Zubair, M.; Al-Harthi, M. Bentonite-layered double hydroxide composite for enhanced aqueous adsorption of Eriochrome Black T. Appl. Clay Sci. 2018, 161, 23–34. [Google Scholar] [CrossRef]
- Guan, X.; Yuan, X.; Zhao, Y.; Bai, J.; Li, Y.; Cao, Y.; Chen, Y.; Xiong, T. Adsorption behaviors and mechanisms of Fe/Mg layered double hydroxide loaded on bentonite on Cd (II) and Pb (II) removal. J. Colloid Interface Sci. 2022, 612, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Kim, H.J.; Oh, J.-M. Interlayer Structure of Bioactive Molecule, 2-Aminoethanesulfonate, Intercalated into Calcium-Containing Layered Double Hydroxides. J. Nanomater. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Haring, M.M. Colloid and capillary chemistry (Freundlich, Herbert). J. Chem. Educ. 1926, 3, 1454. [Google Scholar] [CrossRef] [Green Version]
- Hameed, B.H.; Salman, J.M.; Ahmad, A.L. Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J. Hazard. Mater. 2009, 163, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. A comparative study on the effect of different precursors for synthesis and efficient photocatalytic activity of g-C3N4/TiO2/bentonite nanocomposites. J. Mater. Sci. 2018, 53, 13126–13142. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Sharma, M.; Basu, S. Impact of Ag nanoparticles on photomineralization of chlorobenzene by TiO2/bentonite nanocomposite. J. Environ. Chem. Eng. 2017, 5, 644–651. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, P.; Montavon, G.; Chen, Z.; Wang, D.; Tan, Z.; Jin, Q.; Wu, W.; Wang, J.; Guo, Z. Strengthened erosion resistance of compacted bentonite by layered double hydroxide: A new electrostatic interaction-based approach. Chemosphere 2022, 292, 133402. [Google Scholar] [CrossRef]
- Önal, M.; Sarıkaya, Y. Thermal behavior of a bentonite. J. Therm. Anal. Calorim. 2007, 90, 167–172. [Google Scholar] [CrossRef]
- Makwana, D.; Polisetti, V.; Castaño, J.; Ray, P.; Bajaj, H.C. Mg-Fe layered double hydroxide modified montmorillonite as hydrophilic nanofiller in polysulfone-polyvinylpyrrolidone blend ultrafiltration membranes: Separation of oil-water mixture. Appl. Clay Sci. 2020, 192, 105636. [Google Scholar] [CrossRef]
- Nakayama, S.; Sakamoto, Y.; Yamaguchi, T.; Akai, M.; Tanaka, T.; Sato, T.; Iida, Y. Dissolution of montmorillonite in compacted bentonite by highly alkaline aqueous solutions and diffusivity of hydroxide ions. Appl. Clay Sci. 2004, 27, 53–65. [Google Scholar] [CrossRef]
- Mihaljevič, M.; Ettler, V.; Hradil, D.; Šebek, O.; Strnad, L. Dissolution of bentonite and release of rare earth elements at different solid/liquid ratios in a simulated wine purification process. Appl. Clay Sci. 2006, 31, 36–46. [Google Scholar] [CrossRef]
- Boclair, J.W.; Braterman, P.S. Layered double hydroxide stability. 1. Relative stabilities of layered double hydroxides and their simple counterparts. Chem. Mat. 1999, 11, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Yan, S.; Cheong, S.; Cao, Z.; Zuo, H.; Thomas, A.C.; Rolfe, B.E.; Xu, Z.P. Layered double hydroxide nanoparticles: Impact on vascular cells, blood cells and the complement system. J. Colloid Interface Sci. 2018, 512, 404–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Nguyen, H.Q.; Nguyen, P.T.; Nguyen, D.D.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Mohammed, A.K.; Shuaib, D.T. Potential of using kaolin as a natural adsorbent for the removal of pollutants from tannery wastewater. Heliyon 2019, 5, e02923. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Karn, B.; Kuiken, T.; Otto, M. Nanotechnology and in situ Remediation: A Review of the Benefits and Potential Risks. Environ. Health Perspect. 2009, 117, 1813–1831. [Google Scholar] [CrossRef] [Green Version]
- Jada, A.; Ait Akbour, R.; Douch, J. Surface charge and adsorption from water onto quartz sand of humic acid. Chemosphere 2006, 64, 1287–1295. [Google Scholar] [CrossRef]








| Sample | d-Spacing (nm) (BT) a | FWHM (degrees) (BT) a | d-Spacing (nm) (LDH) b | FWHM (degrees) (LDH) b | Intensity Ratio c | Mg:Al Ratio d |
|---|---|---|---|---|---|---|
| Bentonite | 1.24 | 2.378 | – | – | – | – |
| LDH | – | – | 0.78 | 1.583 | – | 2.13:1 |
| LDH–BT_CP | 1.45 | 0.617 | 0.78 | 1.642 | 4.73 | 1.86:1 * |
| LDH–BT_ER | 1.27 | 0.905 | 0.78 | 1.524 | 10.25 | 1.91:1 * |
| LDH–BT_SP | 0.99 | 0.980 | 0.78 | 1.502 | 1.75 | 2.16:1 * |
| Sample | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | aL (L/mg) | RL | R2 | KF | n | R2 | |
| LDH–BT_SP | 6.705 | 3.191 | 0.331 | 0.8279 | 2.432 | 4.121 | 0.9742 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Son, Y.; Bae, S.; Kim, T.-H.; Hwang, Y. Adsorption of Chromate Ions by Layered Double Hydroxide–Bentonite Nanocomposite for Groundwater Remediation. Nanomaterials 2022, 12, 1384. https://doi.org/10.3390/nano12081384
Kim Y, Son Y, Bae S, Kim T-H, Hwang Y. Adsorption of Chromate Ions by Layered Double Hydroxide–Bentonite Nanocomposite for Groundwater Remediation. Nanomaterials. 2022; 12(8):1384. https://doi.org/10.3390/nano12081384
Chicago/Turabian StyleKim, Yoogyeong, Yeongkyun Son, Sungjun Bae, Tae-Hyun Kim, and Yuhoon Hwang. 2022. "Adsorption of Chromate Ions by Layered Double Hydroxide–Bentonite Nanocomposite for Groundwater Remediation" Nanomaterials 12, no. 8: 1384. https://doi.org/10.3390/nano12081384