Nano Metal-Containing Photocatalysts for the Removal of Volatile Organic Compounds: Doping, Performance, and Mechanisms
Abstract
:1. Introduction
2. Undoped Metal-Containing Photocatalysts for the Removal of VOCs
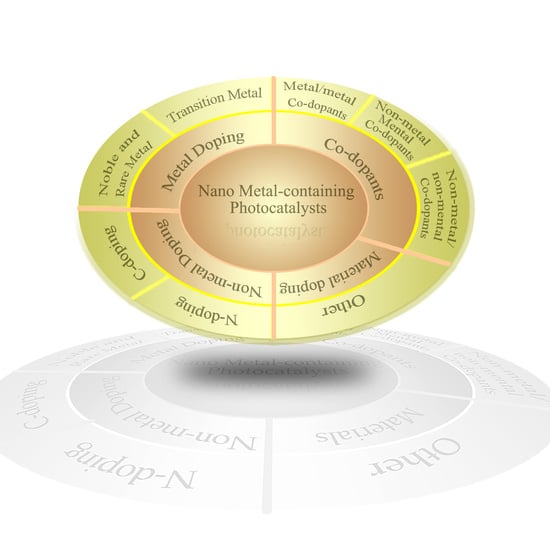
3. Doping of the Metal-Containing Photocatalysts
3.1. Metal-Containing Photocatalysts Doped with Metal
3.1.1. Noble Metal and Rare Metal Doping
3.1.2. Transition Metal Doping
3.2. Metal-Containing Photocatalysts Doped with Non-Metal
3.2.1. N-Doped
3.2.2. C-Doped
3.3. Metal-Cointaining Photocatalysts Doped with Co-Dopants
3.3.1. Metal/Metal Co-Dopants
3.3.2. Non-Metal/Non-Metal Co-Dopants
3.3.3. Non-Metal/Metal Co-Dopants
3.4. Metal-Cointaining Photocatalysts Doped with Other Materials
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wu, Y.J.; Zhang, X.; Cheng, S.; Xiao, W. Analysis on ambient volatile organic compounds and their human gene targets. Aerosol Air Qual. Res. 2018, 18, 2654–2665. [Google Scholar] [CrossRef]
- Lyulyukin, M.N.; Kolinko, P.A.; Selishchev, D.S.; Kozlov, D.V. Hygienic aspects of TiO2-mediated photocatalytic oxidation of volatile organic compounds: Air purification analysis using a total hazard index. Appl. Catal. B 2017, 200, 386–396. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Lin, C.C.; Hsu, S.C. Comparison of conventional and green building materials in respect of VOC emissions and ozone impact on secondary carbonyl emissions. Build. Environ. 2015, 87, 274–282. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Tropospheric air pollution: Ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science 1997, 276, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Szulejko, J.E.; Jo, H.J.; Lee, M.H.; Kim, Y.H.; Kwon, E.; Ma, C.J.; Kumar, P. Measurements of major VOCs released into the closed cabin environment of different automobiles under various engine and ventilation scenarios. Environ. Pollut. 2016, 215, 340–346. [Google Scholar] [CrossRef]
- Wang, W.; Lin, F.; An, T.; Qiu, S.; Yu, H.; Yan, B.; Chen, G.; Hou, L. Photocatalytic mineralization of indoor VOC mixtures over unique ternary TiO2/C/MnO2 with high adsorption selectivity. Chem. Eng. J. 2021, 425, 131678. [Google Scholar] [CrossRef]
- Guieysse, B.; Hort, C.; Platel, V.; Munoz, R.; Ondarts, M.; Revah, S. Biological treatment of indoor air for VOC removal: Potential and challenges. Biotechnol. Adv. 2008, 26, 398–410. [Google Scholar] [CrossRef]
- Gérardin, F.; Cloteaux, A.; Simard, J.; Favre, R. A Photodriven energy efficient membrane process for trace VOC removal from air: First step to a smart approach. Chem. Eng. J. 2021, 419, 129566. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ghfar, A.A. Novel Metal-Organic Framework (MOF) Based Composite Material for the Sequestration of U(VI) and Th(IV) Metal Ions from Aqueous Environment. ACS Appl. Mater. Interfaces 2017, 9, 36026–36037. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Ma, W.L. Photocatalytic oxidation technology for indoor air pollutants elimination: A review. Chemosphere 2021, 280, 130667. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Koshy, P.; Chen, W.F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, S.K.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Wide spectral degradation of Norfloxacin by Ag@BiPO4/BiOBr/BiFeO3 nano-assembly: Elucidating the photocatalytic mechanism under different light sources. J. Hazard. Mater. 2019, 364, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.a.H.; Naushad, M.; Ghfar, A.A.; Guo, C.; Stadler, F.J. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem. Eng. J. 2018, 339, 393–410. [Google Scholar] [CrossRef]
- Zhu, X.D.; Shen, J.; Liu, Y. Removal of formaldehyde and volatile organic compounds from particleboards by air cleaning materials. Adv. Mater. Res. 2010, 113–116, 1870–1873. [Google Scholar] [CrossRef]
- Zhong, L.; Brancho, J.J.; Batterman, S.; Bartlett, B.M.; Godwin, C. Experimental and modeling study of visible light responsive photocatalytic oxidation (PCO) materials for toluene degradation. Appl. Catal. B 2017, 216, 122–132. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Yu, J.Z.; Xu, J.H. Photodegradation of formaldehyde by photocatalyst TiO2: Effects on the presences of NO, SO2 and VOCs. Appl. Catal. B 2004, 54, 41–50. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A review of photocatalysts prepared by sol-gel method for VOCs removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Rao, Z.; Lu, G.; Mahmood, A.; Shi, G.; Xie, X.; Sun, J. Deactivation and activation mechanism of TiO2 and rGO/Er3+-TiO2 during flowing gaseous VOCs photodegradation. Appl. Catal. B 2021, 284, 119813. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]
- Choudhury, B.; Chetri, P.; Choudhury, A. Annealing temperature and oxygen-vacancy-dependent variation of lattice strain, band gap and luminescence properties of CeO2 nanoparticles. J. Exp. Nanosci. 2015, 10, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Weon, S.; Choi, J.; Park, T.; Choi, W. Freestanding doubly open-ended TiO2 nanotubes for efficient photocatalytic degradation of volatile organic compounds. Appl. Catal. B 2017, 205, 386–392. [Google Scholar] [CrossRef]
- El-Roz, M.; Kus, M.; Cool, P.; Thibault-Starzyk, F. New operando IR technique to study the photocatalytic activity and selectivity of TiO2 nanotubes in air purification: Influence of temperature, UV intensity, and VOC concentration. J. Phys. Chem. C 2012, 116, 13252–13263. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; García-Rodríguez, S.; Suárez, S.; Portela, R.; Sánchez, B.; Coronado, J.M. Highly selective one-dimensional TiO2-based nanostructures for air treatment applications. Appl. Catal. B 2011, 110, 251–259. [Google Scholar] [CrossRef]
- Mazhar, S.I.; Shafi, H.Z.; Shah, A.; Asma, M.; Gul, S.; Raffi, M. Synthesis of surface modified hydrophobic PTFE-ZnO electrospun nanofibrous mats for removal of volatile organic compounds (VOCs) from air. Polym. J. 2020, 27, 222. [Google Scholar] [CrossRef]
- Xin, Y.; Chen, Q.; Zhang, G. Construction of ternary heterojunction CuS-CdS/TiO2 nanobelts for photocatalytic degradation of gaseous toluene. J. Alloys Compd. 2018, 751, 231–240. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, M.; Jiang, Y.; Li, J.; Chen, Q. Synthesis of Immobilized CdS/TiO2 Nanofiber Heterostructure Photocatalyst for Efficient Degradation of Toluene. Water Air Soil Pollut. 2020, 231, 92. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Han, D.; Ma, C.; Liu, Y.; Huo, P.; Yan, Y. Bi-based semiconductors composites of BiVO4 quantum dots decorated Bi12TiO20 via in-suit growth with ultrasound for enhancing photocatalytic performance. J. Alloys Compd. 2019, 785, 460–467. [Google Scholar] [CrossRef]
- Li, J.J.; Yu, E.-Q.; Cai, S.C.; Chen, X.; Chen, J.; Jia, H.-P.; Xu, Y.J. Noble metal free, CeO2/LaMnO3 hybrid achieving efficient photo-thermal catalytic decomposition of volatile organic compounds under IR light. Appl. Catal. B 2019, 240, 141–152. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Pu, Y.; Liu, A.; Chen, C.; Zou, W.; Zheng, Y.; Huang, J.; Zhang, Y.; Yang, Y.; et al. Facile ball-milling synthesis of CeO2/g-C3N4 Z-scheme heterojunction for synergistic adsorption and photodegradation of methylene blue: Characteristics, kinetics, models, and mechanisms. Chem. Eng. J. 2021, 420, 127719. [Google Scholar] [CrossRef]
- Mo, J.H.; Zhang, Y.P.; Xu, Q.J.; Lamson, J.J.; Zhao, R.Y. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Enesca, A.; Cazan, C. Volatile Organic Compounds (VOCs) Removal from Indoor Air by Heterostructures/Composites/Doped Photocatalysts: A Mini-Review. Nanomaterials 2020, 10, 1965. [Google Scholar] [CrossRef]
- Thakur, R.S.; Chaudhary, R.; Singh, C. Fundamentals and applications of the photocatalytic treatment for the removal of industrial organic pollutants and effects of operational parameters: A review. J. Renew. Sustain. Energy 2010, 2, 042701. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, P.; Qu, W.; Li, H.; Shi, L.; Zhang, D. Nanodiamond-decorated ZnO catalysts with enhanced photocorrosion-resistance for photocatalytic degradation of gaseous toluene. Appl. Catal. B 2019, 257, 117880. [Google Scholar] [CrossRef]
- Selvam, N.C.; Manikandan, A.; Kennedy, L.J.; Vijaya, J.J. Comparative investigation of zirconium oxide (ZrO2) nano and microstructures for structural, optical and photocatalytic properties. J. Colloid Interface Sci. 2013, 389, 91–98. [Google Scholar] [CrossRef]
- Chung, J.K.; Kim, J.W.; Do, D.; Kim, S.S.; Song, T.K.; Kim, W.J. Energy Band Gap Shift of ZnS-ZnO Thin Films Grown by Pulsed Laser Deposition. Ferroelectrics 2010, 404, 186–191. [Google Scholar] [CrossRef]
- Cai, X.; Zhang, P.; Wei, S.-H. Revisit of the band gaps of rutile SnO2 and TiO2: A first-principles study. J. Semicond. 2019, 40, 092101. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Bakiro, M.; Aljasmi, F.I.A.; Albreiki, A.M.O.; Bayane, S.; Alzamly, A. Investigation of the band gap and photocatalytic properties of CeO2/rGO composites. Mol. Catal. 2020, 486, 110874. [Google Scholar] [CrossRef]
- González-Borrero, P.P.; Sato, F.; Medina, A.N.; Baesso, M.L.; Bento, A.C.; Baldissera, G.; Persson, C.; Niklasson, G.A.; Granqvist, C.G.; da Silva, A.F. Optical band-gap determination of nanostructured WO3 film. Appl. Phys. Lett. 2010, 96, 061909. [Google Scholar] [CrossRef]
- Patidar, D.; Rathore, K.S.; Saxena, N.S.; Sharma, K.; Sharma, T.P. Energy band gap studies of cds nanomaterials. J. Nano Res. 2008, 3, 97–102. [Google Scholar] [CrossRef]
- Deng, J.; Ye, C.; Cai, A.; Huai, L.; Zhou, S.; Dong, F.; Li, X.; Ma, X. S-doping α-Fe2O3 induced efficient electron-hole separation for enhanced persulfate activation toward carbamazepine oxidation: Experimental and DFT study. Chem. Eng. J. 2021, 420, 129863. [Google Scholar] [CrossRef]
- Shiraishi, F.Y.S.; Ohbuchi, Y. A rapid treatment of formaldehyde in a highly tight room using a photocatalytic reactor combined with a continuous adsorption and desorption apparatus. Chem. Eng. Sci. 2003, 58, 929–934. [Google Scholar] [CrossRef]
- Zuo, X.-l.; Wang, S.-f.; Zheng, K.; Wu, C.; Zhang, D.-h.; Dong, Z.-g.; Wang, T.-f.; Xu, F.; Guo, J.-b.; Yang, Y.-y. Fluorescent-brightener-mediated thiol-ene reactions under visible-light LED: A green and facile synthesis route to hyperbranched polymers and stimuli-sensitive nanoemulsions. Dyes Pigm. 2021, 189, 109253. [Google Scholar] [CrossRef]
- Maira, A.J.; Yeung, K.L.; Lee, C.Y.; Yue, P.L.; Chan, C.K. Size effects in gas-phase photo-oxidation of trichloroethylene using nanometer-sized TiO2 catalysts. J. Catal. 2000, 192, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, A.; Ghafourian, H.; Yadegarian, L.; Lari, K.; Sadatipour, M.T. Removal of volatile organic compounds (VOCs) from waste air stream using ozone assisted zinc oxide (ZnO) nanoparticles coated on zeolite. J. Environ. Health Sci. Eng. 2021, 19, 771–780. [Google Scholar] [CrossRef]
- Shayegan, Z.; Haghighat, F.; Lee, C.-S. Anatase/brookite biphasic surface fluorinated Fe–TiO2 photocatalysts to enhance photocatalytic removal of VOCs under visible and UV light. J. Clean. Prod. 2021, 287, 125462. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Yang, R. Novel insight into VOC removal performance of photocatalytic oxidation reactors. Indoor Air 2005, 15, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Xiang, Z.; Li, G.; An, T. Introduce oxygen vacancies into CeO2 catalyst for enhanced coke resistance during photothermocatalytic oxidation of typical VOCs. Appl. Catal. B 2020, 269, 118755. [Google Scholar] [CrossRef]
- Tidahy, H.; Siffert, S.; Lamonier, J.; Zhilinskaya, E.; Aboukais, A.; Yuan, Z.; Vantomme, A.; Su, B.; Canet, X.; Deweireld, G. New Pd/hierarchical macro-mesoporous ZrO2, TiO2 and ZrO2-TiO2 catalysts for VOCs total oxidation. Appl. Catal. A 2006, 310, 61–69. [Google Scholar] [CrossRef]
- Arai, T.; Yanagida, M.; Konishi, Y.; Iwasaki, Y.; Sugihara, H.; Sayama, K. Promotion effect of CuO co-catalyst on WO3-catalyzed photodegradation of organic substances. Catal. Commun. 2008, 9, 1254–1258. [Google Scholar] [CrossRef]
- Paszkiewicz-Gawron, M.; Kowalska, E.; Endo-Kimura, M.; Zwara, J.; Pancielejko, A.; Wang, K.; Lisowski, W.; Łuczak, J.; Zaleska-Medynska, A.; Grabowska-Musiał, E. Stannates, titanates and tantalates modified with carbon and graphene quantum dots for enhancement of visible-light photocatalytic activity. Appl. Surf. Sci. 2021, 541, 11. [Google Scholar] [CrossRef]
- Yang, W.; Cui, W.; Yang, L.; Zhang, G.; Li, X.; Shen, Y.; Dong, F.; Sun, Y. The structural differences of perovskite ATiO3 (A=Ca, Sr) dictate the photocatalytic VOCs mineralization efficiency. Chem. Eng. J. 2021, 425, 130613. [Google Scholar] [CrossRef]
- Fu, X.; Clark, L.A.; Yang, Q.; Anderson, M.A. Enhanced photocatalytic performance of titania-based binary metal oxides: TiO2/SiO2 and TiO2/ZrO2. Environ. Sci. Technol. 1996, 30, 647–653. [Google Scholar] [CrossRef]
- Mendez-Roman, R.; Cardona-Martinez, N. Relationship between the formation of surface species and catalyst deactivation during the gas-phase photocatalytic oxidation of toluene. Catal. Today 1998, 40, 353–365. [Google Scholar] [CrossRef]
- Obuchi, E.; Sakamoto, T.; Nakano, K.; Shiraishi, F. Photocatalytic decomposition of acetaldehyde over TiO2/SiO2 catalyst. Chem. Eng. Sci. 1999, 54, 1525–1530. [Google Scholar] [CrossRef]
- Kamat, P.V.; Meisel, D. Photocatalytic oxidation of acetone vapor on TiO2/ZrO2 thin films. Appl. Catal. B 1999, 23, 1–8. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, J.; Tao, L.; Gong, M.; Zhimin, L.; Chen, Y. Photocatalytic degradation of gaseous benzene over TiO2/Sr2CeO4: Kinetic model and degradation mechanisms. J. Hazard. Mater. 2007, 139, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, B.O.; Newport, K.; Yu, H.; Rownaghi, A.A.; Liang, X.; Rezaei, F. Atomic Layer Deposited Ni/ZrO2-SiO2 for Combined Capture and Oxidation of VOCs. ACS Appl. Mater. Interfaces 2020, 12, 39318–39334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, C.; Ding, J.; Yang, S.; Zang, Y.; Ren, N. One-pot hydrothermal fabrication of BiVO4/Fe3O4/rGO composite photocatalyst for the simulated solar light-driven degradation of Rhodamine B. Front. Environ. Sci. Eng. 2022, 16, 36. [Google Scholar] [CrossRef]
- Wu, H.; Wang, M.; Jing, F.; Kong, D.; Chen, Y.; Jia, C.; Li, J. Enhanced photocatalytic hydrogen production performance of pillararene-doped mesoporous TiO2 with extended visible-light response. Chin. Chem. Lett. 2021, in press. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Albrbar, A.J.; Djokić, V.; Bjelajac, A.; Kovač, J.; Ćirković, J.; Mitrić, M.; Janaćković, D.; Petrović, R. Visible-light active mesoporous, nanocrystalline N, S-doped and co-doped titania photocatalysts synthesized by non-hydrolytic sol-gel route. Ceram. Int. 2016, 42, 16718–16728. [Google Scholar] [CrossRef]
- Khan, R.; Kim, T.J. Preparation and application of visible-light-responsive Ni-doped and SnO2-coupled TiO2 nanocomposite photocatalysts. J. Hazard. Mater. 2009, 163, 1179–1184. [Google Scholar] [CrossRef]
- Barakat, T.; Idakiev, V.; Cousin, R.; Shao, G.S.; Yuan, Z.Y.; Tabakova, T.; Siffert, S. Total oxidation of toluene over noble metal-based Ce, Fe and Ni doped titanium oxides. Appl. Catal. B 2014, 146, 138–146. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, M.; Ai, Z.; Yang, Y.; Xi, H.; Shi, Q.; Xu, X.; Shi, H. Synthesis, characterization, and photocatalytic properties of SnO2/ Rutile TiO2/Anatase TiO2 heterojunctions modified by Pt. J. Phys. Chem. C 2014, 118, 23117–23125. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, W.K. Heterojunction-based two-dimensional N-doped TiO2/WO3 composite architectures for photocatalytic treatment of hazardous organic vapor. J. Hazard. Mater. 2016, 314, 22–31. [Google Scholar] [CrossRef]
- Meisel, K.D. Nanoparticles in advanced oxidation processes. Curr. Opin. Colloid Interface Sci. 2002, 7, 282–287. [Google Scholar] [CrossRef]
- Eun, S.R.; Mavengere, S.; Kim, J.S. Preparation of Ag-TiO2/Sr4Al14O25: Eu2+, Dy3+ photocatalyst on phosphor beads and its photoreaction characteristics. Catalysts 2021, 11, 261. [Google Scholar] [CrossRef]
- Zhan, S.H.; Yang, Y.; Gao, X.C.; Yu, H.B.; Yang, S.S.; Zhu, D.D.; Li, Y. Rapid degradation of toxic toluene using novel mesoporous SiO2 doped TiO2 nanofibers. Catal. Today 2014, 225, 10–17. [Google Scholar] [CrossRef]
- Zang, L.; Macyk, W.; Lange, C.; Maier, W.F.; Antonius, C.; Meissner, D.; Kisch, H. Visible-light detoxification and charge generation by transition metal chloride modified titania. Chemistry 2000, 6, 379–384. [Google Scholar] [CrossRef]
- Bueno-Alejo, C.J.; Graus, J.; Arenal, R.; Lafuente, M.; Bottega-Pergher, B.; Hueso, J.L. Anisotropic Au-ZnO photocatalyst for the visible-light expanded oxidation of n-hexane. Catal. Today 2021, 362, 97–103. [Google Scholar] [CrossRef]
- Burns, A.; Hayes, G.; Li, W.; Hirvonen, J.; Demaree, J.D.; Shah, S.I. Neodymium ion dopant effects on the phase transformation in sol-gel derived titania nanostructures. Mater. Sci. Eng. B 2004, 111, 150–155. [Google Scholar] [CrossRef]
- Yurtsever, H.A.; Ciftcioglu, M. The effect of rare earth element doping on the microstructural evolution of sol-gel titania powders. J. Alloys Compd. 2017, 695, 1336–1353. [Google Scholar] [CrossRef]
- Zhu, W.T.; Lv, Y.Q.; Chen, H.H.; Yang, J.W.; Zhou, X.F. Cadmium and ytterbium Co-doped TiO2 nanorod arrays perovskite solar cells: Enhancement of open circuit voltage and short circuit current density. J. Mater. Sci. Mater. Electron. 2018, 29, 21138–21144. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Peng, K.; Qin, G.; Yang, Y.; Huang, Z. The intrinsic effects of oxygen vacancy and doped non-noble metal in TiO2(B) on photocatalytic oxidation VOCs by visible light driving. J. Environ. Chem. Eng. 2022, 10, 107390. [Google Scholar] [CrossRef]
- Dong, F.; Wang, H.; Wu, Z.; Qiu, J. Marked enhancement of photocatalytic activity and photochemical stability of N-doped TiO2 nanocrystals by Fe3+/Fe2+ surface modification. J. Colloid Interface Sci. 2010, 343, 200–208. [Google Scholar] [CrossRef]
- Saqlain, S.; Cha, B.J.; Kim, S.Y.; Sung, J.Y.; Choi, M.C.; Seo, H.O.; Kim, Y.D. Impact of humidity on the removal of volatile organic compounds over Fe loaded TiO2 under visible light irradiation: Insight into photocatalysis mechanism by operando DRIFTS. Mater. Today Commun. 2021, 26, 102119. [Google Scholar] [CrossRef]
- Hung, W.C.; Chen, Y.C.; Chu, H.; Tseng, T.K. Synthesis and characterization of TiO2 and Fe/TiO2 nanoparticles and their performance for photocatalytic degradation of 1,2-dichloroethane. Appl. Surf. Sci. 2008, 255, 2205–2213. [Google Scholar] [CrossRef]
- Tseng, H.H.; Wei, M.C.; Hsiung, S.F.; Chiou, C.W. Degradation of xylene vapor over Ni-doped TiO2 photocatalysts prepared by polyol-mediated synthesis. Chem. Eng. J. 2009, 150, 160–167. [Google Scholar] [CrossRef]
- Ho, V.T.T.; Chau, D.H.; Bui, K.Q.; Nguyen, N.T.T.; Tran, T.K.N.; Bach, L.G.; Truong, S.N. A high-performing nanostructured Ir doped-TiO2 for efficient photocatalytic degradation of gaseous toluene. Inorganics 2022, 10, 29. [Google Scholar] [CrossRef]
- Jo, W.K.; Kim, J.T. Application of visible-light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds. J. Hazard. Mater. 2009, 164, 360–366. [Google Scholar] [CrossRef]
- Chen, D.M.; Jiang, Z.Y.; Geng, J.Q.; Wang, Q.; Yang, D. Carbon and nitrogen co-doped TiO2 with enhanced visible-light photocatalytic activity. Ind. Eng. Chem. Res. 2007, 46, 2741–2746. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, W.R.; Wu, Z.B. Characterization and photocatalytic activities of C, N and S co-doped TiO2 with 1D nanostructure prepared by the nano-confinement effect. Nanotechnology 2008, 19, 365607. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Zeng, L.; Lu, Z.; Li, M.H.; Yang, J.; Song, W.L.; Zeng, D.W.; Xie, C.S. A modular calcination method to prepare modified N-doped TiO2 nanoparticle with high photocatalytic activity. Appl. Catal. B 2016, 183, 308–316. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Seraphin, S.; Kajitvichyanukul, P. Formation of hydroxyl radicals and kinetic study of 2-chlorophenol photocatalytic oxidation using C-doped TiO2, N-doped TiO2, and C,N Co-doped TiO2 under visible light. Environ. Sci. Pollut. Res. Int. 2016, 23, 3884–3896. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Theory of carbon doping of titanium dioxide. Chem. Mater. 2005, 17, 6656–6665. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K. Selective removal of polar VOCs by novel photocatalytic activity of metals co-doped TiO2/PU under visible light. Chem. Eng. J. 2017, 307, 63–73. [Google Scholar] [CrossRef]
- Neto, N.F.A.; Matsui, K.N.; Paskocimas, C.A.; Bomio, M.R.D.; Motta, F.V. Study of the photocatalysis and increase of antimicrobial properties of Fe3+ and Pb2+ co-doped ZnO nanoparticles obtained by microwave-assisted hydrothermal method. Mater. Sci. Semicond. Process. 2019, 93, 123–133. [Google Scholar] [CrossRef]
- Le Thi Hoang, Y.; Van Thuan, D.; Hanh, N.T.; Vy, N.H.T.; Hang, T.T.M.; Van Ha, H.; Pham, T.-D.; Sharma, A.K.; Nguyen, M.-V.; Dang, N.-M.; et al. Synthesis of N and S Co-doped TiO2 Nanotubes for Advanced Photocatalytic Degradation of Volatile Organic Compounds (VOCs) in Gas Phase. Top. Catal. 2020, 63, 1077–1085. [Google Scholar] [CrossRef]
- Lee, C.-H.; Shie, J.-L.; Yang, Y.-T.; Chang, C.-Y. Photoelectrochemical characteristics, photodegradation and kinetics of metal and non-metal elements co-doped photocatalyst for pollution removal. Chem. Eng. J. 2016, 303, 477–488. [Google Scholar] [CrossRef]
- Zhang, C.B.; Liu, F.D.; Zhai, Y.P.; Ariga, H.; Yi, N.; Liu, Y.C.; Asakura, K.; Flytzani-Stephanopoulos, M.; He, H. Alkali-Metal-Promoted Pt/TiO2 Opens a More Efficient Pathway to Formaldehyde Oxidation at Ambient Temperatures. Angew. Chem. Int. Ed. 2012, 51, 9628–9632. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-light-driven nitrogen-doped TiO2 photocatalysts: Effect of nitrogen precursors on their photocatalysis for decomposition of gas-phase organic pollutants. Mater. Sci. Eng. B 2005, 117, 67–75. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H.; Chen, C.; Li, X.; Niu, M.C.; Gao, X.Y.; Yang, Y.T. Effect of calcination temperature on the structure and visible-light photocatalytic activities of (N, S and C) co-doped TiO2 nano-materials. Appl. Surf. Sci. 2015, 332, 172–180. [Google Scholar] [CrossRef]
- Sirivallop, A.; Escobedo, S.; Areerob, T.; de Lasa, H.; Chiarakorn, S. Photocatalytic conversion of organic pollutants in air: Quantum yields using a silver/nitrogen/TiO2 mesoporous semiconductor under visible light. Catalysts 2021, 11, 529. [Google Scholar] [CrossRef]
- Banisharif, A.; Khodadadi, A.A.; Mortazavi, Y.; Anaraki Firooz, A.; Beheshtian, J.; Agah, S.; Menbari, S. Highly active Fe2O3-doped TiO2 photocatalyst for degradation of trichloroethylene in air under UV and visible light irradiation: Experimental and computational studies. Appl. Catal. B 2015, 165, 209–221. [Google Scholar] [CrossRef]
- Pan, J.H.; Lee, W.I. Preparation of highly ordered cubic mesoporous WO3/TiO2 films and their photocatalytic properties. Chem. Mater. 2006, 18, 847–853. [Google Scholar] [CrossRef]
- Mei, J.; Xie, J.K.; Sun, Y.N.; Qu, Z.; Yan, N.Q. Design of Co3O4/CeO2-Co3O4 hierarchical binary oxides for the catalytic oxidation of dibromomethane. J. Ind. Eng. Chem. 2019, 73, 134–141. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, P.F.; Wang, S.J.; Gao, Y.P.; Chen, F.T.; Zheng, F.; Liu, Y.; Dai, Y.Q. Photocatalytic degradation of gaseous benzene with CdS-sensitized TiO2 film coated on fiberglass cloth. J. Mol. Catal. A 2012, 363, 159–165. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, J.C.; Zhao, X.J. The effect of SiO2 addition on the grain size and photocatalytic activity of TiO2 thin films. J. Sol-Gel Sci. Technol. 2002, 24, 95–103. [Google Scholar] [CrossRef]






| Catalyst | Eg 1 (eV) |
|---|---|
| TiO2 rutile | 3.02 |
| TiO2 anatase | 3.23 |
| ZrO2 | 5.00 |
| ZnS | 3.76 |
| SnO2 | 3.65 |
| ZnO | 3.37 |
| CeO2 | 3.18 |
| WO3 | 2.75 |
| CdS | 2.47 |
| α-Fe2O3 | 2.09 |
| Catalyst | Nanostructure | Synthetic Method | Degraded VOC | Ref |
|---|---|---|---|---|
| P25 * | nanoparticles | flame spray pyrolysis | formaldehyde | [18] |
| P25 * | nanoparticles | flame spray pyrolysis | formaldehyde | [51] |
| TiO2 | nanoparticles | sol-gel technique | TCE | [48] |
| TiO2 | nanotube | potentiostatic anodic oxidation | formaldehyde/toluene | [25] |
| TiO2 | nanotube | hydrothermal method | methanol/n-hexane | [26] |
| TiO2 | nanotube | hydrothermal method | TCE | [27] |
| CeO2 | nanoparticle | redox and steam treatment | n-hexane/cyclohexane | [52] |
| ZrO2 | mesoporous | hydrothermal method | chlorobenzene | [53] |
| WO3 | nanofilm | impregnation method | acetaldehyde | [54] |
| SrTiO3 | nanoparticles | hydrothermal method | toluene | [55] |
| SrTiO3 | nanoparticles | hydrothermal method | toluene | [56] |
| CaTiO3 | nanoparticles | hydrothermal method | toluene | [56] |
| SrSnO3 | nanoparticles | hydrothermal method | toluene | [55] |
| AgTaO3 | nanoparticles | hydrothermal method | toluene | [55] |
| TiO2/CdS | nanobelts | successive ionic layer adsorption and reaction method | toluene | [29] |
| TiO2/CdS | nanofiber | anodic oxidation. | toluene | [30] |
| TiO2/ZrO2 | nanoparticles | sol-gel technique | ethylene | [57] |
| TiO2/SiO2 | nanoparticles | sol-gel technique | ethylene | [57] |
| TiO2/SiO2 | nanoparticles | hydrolysis | toluene | [58] |
| TiO2/SiO2 | nanoparticles | impregnation method | acetaldehyde | [59] |
| TiO2/ZrO2 | nanofilms | sol-gel technique | acetone | [60] |
| TiO2/Sr2CeO4 | nanoparticles | grinding and heating | benzene | [61] |
| TiO2/C/MnO2 | nanoparticles | solvothermal method | formaldehyde/toluene | [8] |
| ZnO/PTFE | nanofibers | electrospinning | toluene/formaldehyde/acetone | [28] |
| ZnO/zeolite | nanoparticles | chemical co-precipitation | benzene series | [49] |
| ZrO2-SiO2 | nanoparticles | atomic layer deposition method | benzene | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, R.; Xia, J.; Wen, J.; Xu, P.; Zheng, X. Nano Metal-Containing Photocatalysts for the Removal of Volatile Organic Compounds: Doping, Performance, and Mechanisms. Nanomaterials 2022, 12, 1335. https://doi.org/10.3390/nano12081335
Cheng R, Xia J, Wen J, Xu P, Zheng X. Nano Metal-Containing Photocatalysts for the Removal of Volatile Organic Compounds: Doping, Performance, and Mechanisms. Nanomaterials. 2022; 12(8):1335. https://doi.org/10.3390/nano12081335
Chicago/Turabian StyleCheng, Rong, Jincheng Xia, Junying Wen, Pingping Xu, and Xiang Zheng. 2022. "Nano Metal-Containing Photocatalysts for the Removal of Volatile Organic Compounds: Doping, Performance, and Mechanisms" Nanomaterials 12, no. 8: 1335. https://doi.org/10.3390/nano12081335