A Facile and General Approach to Enhance Water Resistance of Metal-Organic Frameworks by the Post-Modification with Aminopropyltriethoxylsilane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CuBTC
2.3. Preparation of CuBTC@APTES
2.4. Characterization
3. Results
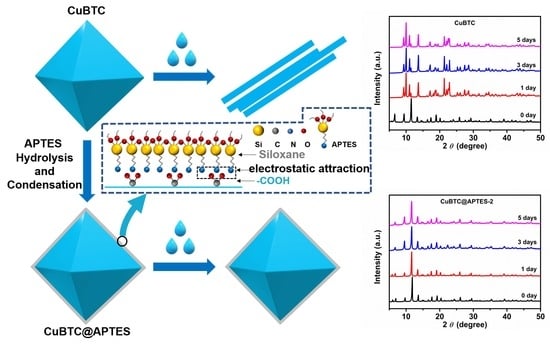
3.1. Synthesis and Characterization of CuBTC and CuBTC@APTES
3.2. Water Stability of CuBTC and CuBTC@APTES
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, X.-J.; Li, Z.-X.; Xue, H.; Huang, X.; Cao, R.; Liu, T.-F. Designing a Bifunctional Brønsted Acid–Base Heterogeneous Catalyst Through Precise Installation of Ligands on Metal–Organic Frameworks. CCS Chem. 2020, 2, 616–622. [Google Scholar] [CrossRef]
- Liu, D.; Wan, J.; Pang, G.; Tang, Z. Hollow Metal-Organic-Framework Micro/Nanostructures and their Derivatives: Emerging Multifunctional Materials. Adv. Mater. 2019, 31, e1803291. [Google Scholar] [CrossRef] [PubMed]
- Heinke, L.; Wöll, C. Surface-Mounted Metal-Organic Frameworks: Crystalline and Porous Molecular Assemblies for Fundamental Insights and Advanced Applications. Adv. Mater. 2019, 31, e1806324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal-Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.-G.; Zhang, G.-Y.; Hu, T.-L.; Bu, X.-H. Metal-organic framework-based heterogeneous catalysts for the conversion of C1 chemistry: CO, CO2 and CH4. Coord. Chem. Rev. 2019, 387, 79–120. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, Y.; Shi, Y.; Li, M.; Li, J.; Duan, C. Modulating photoelectronic performance of metal–organic frameworks for premium photocatalysis. Coord. Chem. Rev. 2019, 380, 201–229. [Google Scholar] [CrossRef]
- Gong, W.; Liu, Y.; Li, H.; Cui, Y. Metal-organic frameworks as solid Brønsted acid catalysts for advanced organic transformations. Coord. Chem. Rev. 2020, 420, 213400. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Xiao, J.-D.; Jiang, H.-L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Acc. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Huang, X.; Chen, Y.; Wang, Y.-R.; Zhang, H.; Li, C.-X.; Zhang, L.; Zhu, H.; Yang, R.; Kan, Y.-H.; et al. Polyoxometalate-Induced Efficient Recycling of Waste Polyester Plastics into Metal–Organic Frameworks. CCS Chem. 2019, 1, 561–570. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, T.; Wu, P.; Huang, J.; Li, H.; Khalil, I.E.; Li, S.; Zheng, B.; Wu, J.; Wang, Q.; et al. Regulating Electronic Status of Platinum Nanoparticles by Metal–Organic Frameworks for Selective Catalysis. CCS Chem. 2021, 3, 1607–1614. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Tian, J.-W.; Liu, S.; Li, B.; Zhao, J.; Ma, L.-F.; Li, D.-S.; Lan, Y.; Bu, X. Bi-Microporous Metal-Organic Frameworks with Cubane [M4(OH)4] (M=Ni, Co) Clusters and Pore-Space Partition for Electrocatalytic Methanol Oxidation Reaction. Angew. Chem. Int. Ed. 2019, 58, 12185–12189. [Google Scholar] [CrossRef]
- Noor, T.; Pervaiz, S.; Iqbal, N.; Sharif, M.; Pervaiz, E. Nanocomposites of NiO/CuO Based MOF with rGO: An Efficient and Robust Electrocatalyst for Methanol Oxidation Reaction in DMFC. Nanomaterials 2020, 10, 1601. [Google Scholar] [CrossRef] [PubMed]
- Grajciar, L.; Bludský, O.; Nachtigall, P. Water Adsorption on Coordinatively Unsaturated Sites in CuBTC MOF. J. Phys. Chem. Lett. 2010, 1, 3354–3359. [Google Scholar] [CrossRef]
- Chui, S.S.-Y.; Lo, S.M.-F.; Charmant, J.P.H.; Orpen, A.G.; WiLLiams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Xin, C.; Jiao, X.; Yin, Y.; Zhan, H.; Li, H.; Li, L.; Zhao, N.; Xiao, F.; Wei, W. Enhanced CO2 Adsorption Capacity and Hydrothermal Stability of HKUST-1 via Introduction of Siliceous Mesocellular Foams (MCFs). Ind. Eng. Chem. Res. 2016, 55, 7950–7957. [Google Scholar] [CrossRef]
- Jia, J.; Wang, Y.; Feng, Y.; Hu, G.; Lin, J.; Huang, Y.; Zhang, Y.; Liu, Z.; Tang, C.; Yu, C. Hierarchically Porous Boron Nitride/HKUST-1 Hybrid Materials: Synthesis, CO2 Adsorption Capacity, and CO2/N2 and CO2/CH4 Selectivity. Ind. Eng. Chem. Res. 2021, 60, 2463–2471. [Google Scholar] [CrossRef]
- Cortés-Súarez, J.; Celis-Arias, V.; Beltrán, H.I.; Tejeda-Cruz, A.; Ibarra, I.A.; Romero-Ibarra, J.E.; Sánchez-González, E.; Loera-Serna, S. Synthesis and Characterization of an SWCNT@HKUST-1 Composite: Enhancing the CO2 Adsorption Properties of HKUST-1. ACS Omega 2019, 4, 5275–5282. [Google Scholar] [CrossRef] [Green Version]
- Terracina, A.; McHugh, L.N.; Todaro, M.; Agnello, S.; Wheatley, P.S.; Gelardi, F.M.; Morris, R.E.; Buscarino, G. Multitechnique Analysis of the Hydration in Three Different Copper Paddle-Wheel Metal–Organic Frameworks. J. Phys. Chem. C 2019, 123, 28219–28232. [Google Scholar] [CrossRef]
- Luo, Q.-X.; Song, X.-D.; Ji, M.; Park, S.-E.; Hao, C.; Li, Y.-Q. Molecular size- and shape-selective Knoevenagel condensation over microporous Cu3(BTC)2 immobilized amino-functionalized basic ionic liquid catalyst. Appl. Catal. A-Gen. 2014, 478, 81–90. [Google Scholar] [CrossRef]
- Lu, X.F.; Xia, B.Y.; Zang, S.-Q.; Lou, X.W.D. Metal-Organic Frameworks Based Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 59, 4634–4650. [Google Scholar] [CrossRef] [PubMed]
- Vrtovec, N.; Mazaj, M.; Buscarino, G.; Terracina, A.; Agnello, S.; Arčon, I.; Kovač, J.; Logar, N.Z. Structural and CO2 Capture Properties of Ethylenediamine-Modified HKUST-1 Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 5455–5465. [Google Scholar] [CrossRef]
- Sharma, D.; Rasaily, S.; Pradhan, S.; Baruah, K.; Tamang, S.; Pariyar, A. HKUST-1 Metal Organic Framework as an Efficient Dual-Function Catalyst: Aziridination and One-Pot Ring-Opening Transformation for Formation of beta-Aryl Sulfonamides with C-C, C-N, C-S, and C-O Bonds. Inorg. Chem. 2021, 60, 7794–7802. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Liu, S.; Chen, Y.; Cheang, U.K.; George, M.W.; Wu, T. Mechanistic and Experimental Study of the Formation of MoS2/HKUST-1 Core–Shell Composites on MoS2 Quantum Dots with an Enhanced CO2 Adsorption Capacity. Ind. Eng. Chem. Res. 2020, 59, 5808–5817. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Demir, N.K.; Chenbc, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sharma, S.; Ghosh, S.K. Hydrophobic metal-organic frameworks: Potential toward emerging applications. APL Mater. 2019, 7, 050701. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Li, J.; Guan, B.; Jia, M.; Terasaki, O.; Yu, J. A Green Selective Water-Etching Approach to MOF@Mesoporous SiO2 Yolk-Shell Nanoreactors with Enhanced Catalytic Stabilities. Matter 2020, 3, 498–508. [Google Scholar] [CrossRef]
- Wang, C.; An, B.; Lin, W. Metal–Organic Frameworks in Solid–Gas Phase Catalysis. ACS Catal. 2018, 9, 130–146. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Zhang, X.-W.; Mo, Z.-W.; Xu, Y.-Z.; Tian, X.-Y.; Li, Y.; Chen, X.-M.; Zhang, J.-P. Adsorptive separation of carbon dioxide: From conventional porous materials to metal–organic frameworks. EnergyChem 2019, 1, 100016. [Google Scholar] [CrossRef]
- Xie, L.-H.; Xu, M.-M.; Liu, X.-M.; Zhao, M.-J.; Li, J.-R. Hydrophobic Metal-Organic Frameworks: Assessment, Construction, and Diverse Applications. Adv. Sci. 2020, 7, 1901758. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Catalysis by metal-organic frameworks in water. Chem. Commun. 2014, 50, 12800–12814. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Jiang, H.-L. Improving Water Stability of Metal–Organic Frameworks by a General Surface Hydrophobic Polymerization. CCS Chem. 2021, 3, 2740–2748. [Google Scholar] [CrossRef]
- Barbara, S.; Andreas, M.; Thomas, H. Interaction of Biologically Important Organic Molecules with the Unsaturated Copper Centers of the HKUST-1 Metal−Organic Framework: An Ab-Initio Study. J. Phys. Chem. C 2015, 119, 3024–3032. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Geyer, F.; Schneemann, A.; Kment, S.; Otyepka, M.; Zboril, R.; Vollmer, D.; Fischer, R.A. Hydrophobic Metal-Organic Frameworks. Adv. Mater. 2019, 31, e1900820. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Mileo, P.G.M.; Bok, I.; Peh, S.B.; Zhang, J.; Yuan, H.; Maurin, G.; Zhao, D. Thermo-Responsive MOF/Polymer Composites for Temperature-Mediated Water Capture and Release. Angew. Chem. Int. Ed. 2020, 59, 11003–11009. [Google Scholar] [CrossRef]
- Terracina, A.; Todaro, M.; Mazaj, M.; Agnello, S.; Gelardi, F.M.; Buscarino, G. Unveiled the Source of the Structural Instability of HKUST-1 Powders upon Mechanical Compaction: Definition of a Fully Preserving Tableting Method. J. Phys. Chem. C 2018, 123, 1730–1741. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lin, Z.; Zhou, X.; Wang, X.; Li, Y.; Wang, H.; Li, Z. Ultrafast room temperature synthesis of novel composites Imi@Cu-BTC with improved stability against moisture. Chem. Eng. J. 2017, 307, 537–543. [Google Scholar] [CrossRef]
- Yang, L.; Idrees, K.B.; Chen, Z.; Knapp, J.; Chen, Y.; Wang, X.; Cao, R.; Zhang, X.; Xing, H.; Islamoglu, T.; et al. Nanoporous Water-Stable Zr-Based Metal–Organic Frameworks for Water Adsorption. ACS Appl. Nano Mater. 2021, 4, 4346–4350. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Schindler, B.J.; Killops, K.L.; Browe, M.A.; Mahle, J.J. The effect of water adsorption on the structure of the carboxylate containing metal–organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A 2013, 1, 11922. [Google Scholar] [CrossRef]
- Antwi-Baah, R.; Liu, H. Recent Hydrophobic Metal-Organic Frameworks and Their Applications. Materials 2018, 11, 2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Fang, R.; Luque, R.; Chen, L.; Li, Y. Functional metal–organic frameworks for catalytic applications. Coord. Chem. Rev. 2019, 388, 268–292. [Google Scholar] [CrossRef]
- Sun, D.; Adiyala, P.R.; Yim, S.J.; Kim, D.P. Pore Surface Engineering by Decorating Metal-oxo Nodes with Phenylsilane for Versatile Super-Hydrophobic Metal-Organic Frameworks (MOFs). Angew. Chem. Int. Ed. 2019, 58, 7405–7409. [Google Scholar] [CrossRef]
- He, S.; Wang, H.; Zhang, C.; Zhang, S.; Yu, Y.; Lee, Y.; Li, T. A generalizable method for the construction of MOF@polymer functional composites through surface-initiated atom transfer radical polymerization. Chem. Sci. 2019, 10, 1816–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yagh, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal-organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cahil, J.F.; Fe, H.; Prather, K.A.; Cohen, S.M. Postsynthetic ligand and cation exchange in robust metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 18082–18088. [Google Scholar] [CrossRef] [PubMed]
- Al-Janabi, N.; Deng, H.; Borges, J.; Liu, X.; Garforth, A.; Siperstein, F.R.; Fan, X. A Facile Post-Synthetic Modification Method to Improve Hydrothermal Stability and CO2 Selectivity of CuBTC Metal–Organic Framework. Ind. Eng. Chem. Res. 2016, 55, 7941–7949. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Hu, Y.; Ge, J.; Jiang, H.L.; Yu, S.H. A facile and general coating approach to moisture/water-resistant metal-organic frameworks with intact porosity. J. Am. Chem. Soc. 2014, 136, 16978–16981. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Yu, F.; Liu, H.; Zhou, K.; Wei, J.; Huang, Y. MOF-templated formation of porous CuO hollow octahedra for lithium-ion battery anode materials. J. Mater. Chem. A 2013, 1, 11126. [Google Scholar] [CrossRef]
- Qian, X.; Sun, F.; Sun, J.; Wu, H.; Xiao, F.; Wu, X.; Zhu, G. Imparting surface hydrophobicity to metal-organic frameworks using a facile solution-immersion process to enhance water stability for CO2 capture. Nanoscale 2017, 9, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Grzech, A.; Yang, J.; Dingemans, T.J.; Srinivasan, S.; Magusin, P.C.M.M.; Mulder, F.M. Irreversible High-Temperature Hydrogen Interaction with the Metal Organic Framework Cu3(BTC)2. J. Phys. Chem. C 2011, 115, 21521–21525. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Jie, X.; Liu, D.; Cao, Y. Improved Interfacial Affinity and CO2 Separation Performance of Asymmetric Mixed Matrix Membranes by Incorporating Postmodified MIL-53(Al). ACS Appl. Mater. Interfaces 2016, 8, 22696–22704. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Li, J.; Ma, Q. A Facile and General Approach to Enhance Water Resistance of Metal-Organic Frameworks by the Post-Modification with Aminopropyltriethoxylsilane. Nanomaterials 2022, 12, 1134. https://doi.org/10.3390/nano12071134
Gu J, Li J, Ma Q. A Facile and General Approach to Enhance Water Resistance of Metal-Organic Frameworks by the Post-Modification with Aminopropyltriethoxylsilane. Nanomaterials. 2022; 12(7):1134. https://doi.org/10.3390/nano12071134
Chicago/Turabian StyleGu, Jianmei, Jianquan Li, and Qingyu Ma. 2022. "A Facile and General Approach to Enhance Water Resistance of Metal-Organic Frameworks by the Post-Modification with Aminopropyltriethoxylsilane" Nanomaterials 12, no. 7: 1134. https://doi.org/10.3390/nano12071134