Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review
Abstract
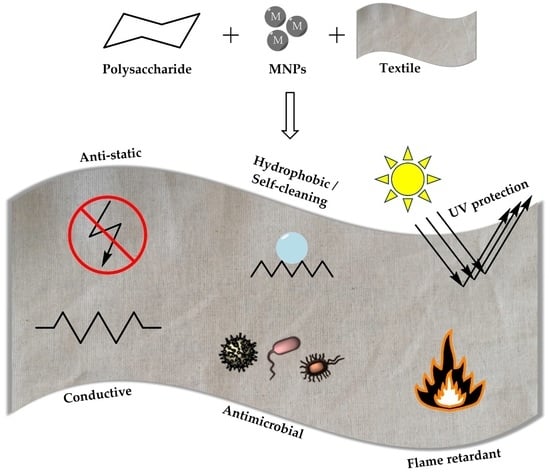
:1. Introduction
2. Polysaccharides in Metal-Nanoparticle-Functionalized Textiles
2.1. Chitosan
2.1.1. Substrate Composition
2.1.2. Enhancing the Adhesion of Metal Nanoparticles onto Textiles and/or Controlling the Release of Nanoparticles or Metal Ions
2.1.3. Multifunctional Textiles
2.1.4. Action as a Reducing Agent of Metal Salts and Stabilization of Metal Nanoparticles on Dispersions
2.2. Alginate
2.3. Starch
2.4. Cyclodextrins
2.5. Cellulose
2.6. Other Polysaccharides
3. Safety Issues of MNPs and the Role of Polysaccharides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Modification of textiles for functional applications. In Fundamentals of Natural Fibres and Textiles; Mondal, M.I.H., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 303–365. [Google Scholar]
- Emam, H.E. Generic strategies for functionalization of cellulosic textiles with metal salts. Cellulose 2018, 26, 1431–1447. [Google Scholar] [CrossRef]
- Singh, M.K. Textiles Functionalization—A Review of Materials, Processes, and Assessment. In Textiles for Functional Applications; Kumar, B., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Bhattacharyya, A. Nanotechnology—A new route to high-performance functional textiles. Text. Prog. 2011, 43, 155–233. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Mehravani, B.; Ribeiro, A.; Zille, A. Gold Nanoparticles Synthesis and Antimicrobial Effect on Fibrous Materials. Nanomaterials 2021, 11, 1067. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; De Vrieze, M.; Felgueiras, H.P.; et al. Effect of Dispersion Solvent on the Deposition of PVP-Silver Nanoparticles onto DBD Plasma-Treated Polyamide 6,6 Fabric and Its Antimicrobial Efficiency. Nanomaterials 2020, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Rivero, P.J.; Urrutia, A.; Goicoechea, J.; Arregui, F.J. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res. Lett. 2015, 10, 501. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.I.; Senturk, D.; Silva, K.S.; Modic, M.; Cvelbar, U.; Dinescu, G.; Mitu, B.; Nikiforov, A.; Leys, C.; Kuchakova, I.; et al. Efficient silver nanoparticles deposition method on DBD plasma-treated polyamide 6,6 for antimicrobial textiles. IOP Conf. Ser. Mater. Sci. Eng. 2018, 460, 012007. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S. Sustainable Use of Nanomaterials in Textiles and Their Environmental Impact. Materials 2020, 13, 5134. [Google Scholar] [CrossRef]
- Riaz, S.; Ashraf, M.; Hussain, T.; Hussain, M.T.; Rehman, A.; Javid, A.; Iqbal, K.; Basit, A.; Aziz, H. Functional finishing and coloration of textiles with nanomaterials. Coloration Technol. 2018, 134, 327–346. [Google Scholar] [CrossRef]
- Xu, Q.; Ke, X.; Shen, L.; Ge, N.; Zhang, Y.; Fu, F.; Liu, X. Surface modification by carboxymethy chitosan via pad-dry-cure method for binding Ag NPs onto cotton fabric. Int. J. Biol. Macromol. 2018, 111, 796–803. [Google Scholar] [CrossRef]
- Mohamed, A.L.; Hassabo, A.G. Composite material based on pullulan/silane/ZnO-NPs as pH, thermo-sensitive and antibacterial agent for cellulosic fabrics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 045005. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.-X.; Zhang, B.; Ramakrishna, S.; Yu, M.; Ma, J.-W.; Long, Y.-Z. In Situ Assembly of Well-Dispersed Ag Nanoparticles throughout Electrospun Alginate Nanofibers for Monitoring Human Breath—Smart Fabrics. ACS Appl. Mater. Interfaces 2018, 10, 19863–19870. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Li, J.; He, B.; Sun, J.; Li, L.; Qian, L. Novel Wearable Electrodes Based on Conductive Chitosan Fabrics and Their Application in Smart Garments. Materials 2018, 11, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, M.M.; Rehan, M.; El-Sheikh, S.M.; Zahran, M.K.; Abdel-Aziz, M.S.; Bechelany, M.; Barhoum, A. Multifunctional Hydroxyapatite/Silver Nanoparticles/Cotton Gauze for Antimicrobial and Biomedical Applications. Nanomaterials 2021, 11, 429. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Fernandes, M.; Souto, A.P.; Dourado, F.; Gama, M. Application of Bacterial Cellulose in the Textile and Shoe Industry: Development of Biocomposites. Polysaccharides 2021, 2, 34. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, J.; Lu, Z.; Wei, H.; Yu, Y. Natural Polysaccharides as Multifunctional Components for One-Step 3D Printing Tough Hydrogels. Macromol. Mater. Eng. 2021, 306, 2100433. [Google Scholar] [CrossRef]
- Poshina, D.; Otsuka, I. Electrospun Polysaccharidic Textiles for Biomedical Applications. Textiles 2021, 1, 7. [Google Scholar] [CrossRef]
- Fernandes, M.; Gama, M.; Dourado, F.; Souto, A.P. Development of novel bacterial cellulose composites for the textile and shoe industry. Microb. Biotechnol. 2019, 12, 650–661. [Google Scholar] [CrossRef] [Green Version]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Dourado, F. Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll. 2016, 58, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Mongkholrattanasit, R.; Klaichoi, C.; Rungruangkitkrai, N. Printing Silk Fabric with Acid Dye Using a New Thickening Agent. J. Nat. Fibers 2021, in press. [Google Scholar] [CrossRef]
- Singh, N.; Sahu, O. Sustainable cyclodextrin in textile applications. In The Impact and Prospects of Green Chemistry for Textile Technology; Islam, S., Butola, B.S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 83–105. [Google Scholar]
- Chen, F.; Huang, G. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jia, Y.; Guo, Q. Polysaccharides and polysaccharide complexes as potential sources of antidiabetic compounds: A review. In Bioactive Natural Products; Atta-ur-Rahman, Ed.; Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–220. [Google Scholar]
- De Jesus Raposo, M.; de Morais, A.; de Morais, R. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Niu, Y.; Xing, P.; Wang, C. Bioactive polysaccharides from natural resources including Chinese medicinal herbs on tissue repair. Chin. Med. 2018, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-C.; Tang, I.C. Bacterial and Yeast Cultures—Process Characteristics, Products, and Applications. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 185–223. [Google Scholar]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials—The Place of Inorganics-in-Organics in it, Their Composition and Applications. Front. Chem. 2019, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Lizundia, E.; Puglia, D.; Nguyen, T.-D.; Armentano, I. Cellulose nanocrystal based multifunctional nanohybrids. Prog. Mater. Sci. 2020, 112, 100668. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.; Silvestre, A.; da Rocha Freire Barros, C.S. Polysaccharides-Based Hybrids with Metal Nanoparticles. In Polysaccharide Based Hybrid Materials; Springer Briefs in Molecular Science; Springer: Cham, Switzerland, 2018; pp. 9–30. [Google Scholar]
- Wang, C.; Gao, X.; Chen, Z.; Chen, Y.; Chen, H. Preparation, Characterization and Application of Polysaccharide-Based Metallic Nanoparticles: A Review. Polymers 2017, 9, 689. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Su, C.; Jiang, L.; Ye, S.; Liu, X.; Shao, W. Green and Facile Preparation of Chitosan Sponges as Potential Wound Dressings. ACS Sustain. Chem. Eng. 2018, 6, 9145–9152. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M.; Sobahi, T.R.A. Chitosan coated cotton cloth supported zero-valent nanoparticles: Simple but economically viable, efficient and easily retrievable catalysts. Sci. Rep. 2017, 7, 16957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.-S.; Zhang, Y.; Fang, T.; Han, Z.-B.; Liang, F.-S. Chitosan-Coated Metal–Organic-Framework Nanoparticles as Catalysts for Tandem Deacetalization–Knoevenagel Condensation Reactions. ACS Appl. Nano Mater. 2020, 3, 6316–6320. [Google Scholar] [CrossRef]
- Shoueir, K.; Kandil, S.; El-hosainy, H.; El-Kemary, M. Tailoring the surface reactivity of plasmonic Au@TiO2 photocatalyst bio-based chitosan fiber towards cleaner of harmful water pollutants under visible-light irradiation. J. Clean. Prod. 2019, 230, 383–393. [Google Scholar] [CrossRef]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and Properties of Chitosan Inks for 3D Printing of Hydrogel Microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef]
- Panzarasa, G.; Osypova, A.; Sicher, A.; Bruinink, A.; Dufresne, E.R. Controlled formation of chitosan particles by a clock reaction. Soft Matter 2018, 14, 6415–6418. [Google Scholar] [CrossRef]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biol. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef]
- Bayat Tork, M.; Hemmati Nejad, N.; Ghalehbagh, S.; Bashari, A.; Shakeri-Zadeh, A.; Kamrava, S.K. In situ green synthesis of silver nanoparticles/chitosan/poly vinyl alcohol/poly ethylene glycol hydrogel nanocomposite for novel finishing of nasal tampons. J. Ind. Text. 2014, 45, 1399–1416. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Wang, H.; Mahmud, S.; Genyang, C. Coloration of aramid fabric via in-situ biosynthesis of silver nanoparticles with enhanced antibacterial effect. Inorg. Chem. Commun. 2020, 119, 108115. [Google Scholar] [CrossRef]
- Ramadan, M.A.; Sharawy, S.; Elbisi, M.K.; Ghosal, K. Eco-friendly Packaging Composite Fabrics based on in situ synthesized Silver nanoparticles (AgNPs) & treatment with Chitosan and/or Date seed extract. Nano-Struct. Nano-Objects 2020, 22, 100425. [Google Scholar] [CrossRef]
- Ramadan, M.A.; Taha, G.M.; El-Mohr, W.Z.E.-A. Antimicrobial and UV protection finishing of Polysaccharide -Based Textiles using Biopolymer and AgNPs. Egypt. J. Chem. 2020, 63, 2707–2716. [Google Scholar] [CrossRef]
- Shakeri-Zadeh, A.; Bashari, A.; Kamrava, S.K.; Ghalehbaghi, S. The Use of Hydrogel/Silver Nanoparticle System for Preparation of New Type of Feminine Tampons. BioNanoScience 2016, 6, 284–292. [Google Scholar] [CrossRef]
- Montaser, A.S.; Mahmoud, F.A. Preparation of Chitosan-grafted-Polyvinyl acetate Metal Nanocomposite for producing Multifunctional Textile Cotton Fabrics. Int. J. Biol. Macromol. 2019, 124, 659–666. [Google Scholar] [CrossRef]
- Mogrovejo-Valdivia, A.; Rahmouni, O.; Tabary, N.; Maton, M.; Neut, C.; Martel, B.; Blanchemain, N. In vitro evaluation of drug release and antibacterial activity of a silver-loaded wound dressing coated with a multilayer system. Int. J. Pharm. 2019, 556, 301–310. [Google Scholar] [CrossRef]
- Zayed, M.; Othman, H.; Ghazal, H.; Hassabo, A.G. Psidium Guajava Leave Extract as Reducing Agent for Synthesis of Zinc Oxide Nanoparticles and its Application to Impart Multifunctional Properties for Cellulosic Fabrics. Biointerface Res. Appl. Chem. 2021, 11, 13535–13556. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, J.; Wang, X. Hydrophilic and antimicrobial properties of acrylic acid and chitosan bigrafted polypropylene melt-blown nonwoven membrane immobilized with silver nanoparticles. Text. Res. J. 2016, 88, 182–190. [Google Scholar] [CrossRef]
- Hatami, M.; Sharifi, A.; Karimi-Maleh, H.; Agheli, H.; Karaman, C. Simultaneous improvements in antibacterial and flame retardant properties of PET by use of bio-nanotechnology for fabrication of high performance PET bionanocomposites. Environ. Res. 2022, 206, 112281. [Google Scholar] [CrossRef]
- Rehan, M.; El-Naggar, M.E.; Mashaly, H.M.; Wilken, R. Nanocomposites based on chitosan/silver/clay for durable multi-functional properties of cotton fabrics. Carbohydr. Polym. 2018, 182, 29–41. [Google Scholar] [CrossRef]
- Montaser, A.S.; Jlassi, K.; Ramadan, M.A.; Sleem, A.A.; Attia, M.F. Alginate, gelatin, and carboxymethyl cellulose coated nonwoven fabrics containing antimicrobial AgNPs for skin wound healing in rats. Int. J. Biol. Macromol. 2021, 173, 203–210. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, X.; Zhu, Y.; Lyu, H.; Zhang, L.; Fu, F.; Liu, X. A hybrid binder of carboxymethyl chitosan and l-methionine enables a slight amount of Ag NPs to be durably effective on antibacterial cotton fabrics. Cellulose 2019, 26, 9323–9333. [Google Scholar] [CrossRef]
- Türemen, M.; Demir, A.; Gokce, Y. The synthesis and application of chitosan coated ZnO nanorods for multifunctional cotton fabrics. Mater. Chem. Phys. 2021, 268, 124736. [Google Scholar] [CrossRef]
- Saleh, S.N.; Khaffaga, M.M.; Ali, N.M.; Hassan, M.S.; El-Naggar, A.W.M.; Rabie, A.G.M. Antibacterial functionalization of cotton and cotton/polyester fabrics applying hybrid coating of copper/chitosan nanocomposites loaded polymer blends via gamma irradiation. Int. J. Biol. Macromol. 2021, 183, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Kołodziejczyk, M.; Szynkowska, M.; Pawlaczyk, A.; Leśniewska, E.; Matusiak, A.; Adamus, A.; Karolczak, A. Hydrogels made from chitosan and silver nitrate. Carbohydr. Polym. 2016, 140, 74–87. [Google Scholar] [CrossRef]
- Hajimirzababa, H.; Khajavi, R.; Mirjalili, M.; KarimRahimi, M. Modified cotton gauze with nano-Ag decorated alginate microcapsules and chitosan loaded with PVP-I. J. Text. Inst. 2017, 109, 677–685. [Google Scholar] [CrossRef]
- Sheikh, J.; Bramhecha, I. Multi-functionalization of linen fabric using a combination of chitosan, silver nanoparticles and Tamarindus indica L. seed coat extract. Cellulose 2019, 26, 8895–8905. [Google Scholar] [CrossRef]
- Awais; Ali, N.; Khan, A.; Asiri, A.M.; Kamal, T. Potential application of in-situ synthesized cobalt nanoparticles on chitosan-coated cotton cloth substrate as catalyst for the reduction of pollutants. Environ. Technol. Innov. 2021, 23, 101675. [Google Scholar] [CrossRef]
- Ali, N.; Awais; Kamal, T.; Ul-Islam, M.; Khan, A.; Shah, S.J.; Zada, A. Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int. J. Biol. Macromol. 2018, 111, 832–838. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Abd El-Ghany, N.A.; Mabrouk, E.M. Polyfunctional cotton cellulose fabric using proper biopolymers and active ingredients. J. Text. Inst. 2019, 111, 381–393. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; El-Aziz, E.A.; Elmaaty, T.M.A.; Ramadan, S.M. Loading of chitosan—Nano metal oxide hybrids onto cotton/polyester fabrics to impart permanent and effective multifunctions. Int. J. Biol. Macromol. 2017, 105, 769–776. [Google Scholar] [CrossRef]
- Yu, X.; Pan, Y.; Wang, D.; Yuan, B.; Song, L.; Hu, Y. Fabrication and Properties of Biobased Layer-by-Layer Coated Ramie Fabric-Reinforced Unsaturated Polyester Resin Composites. Ind. Eng. Chem. Res. 2017, 56, 4758–4767. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Wang, H.; Mahmud, S.; Jahid, M.A.; Islam, M.; Jin, W.; Genyang, C. Colorful and antibacterial nylon fabric via in-situ biosynthesis of chitosan mediated nanosilver. J. Mater. Res. Technol. 2020, 9, 16135–16145. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Luyt, A.S. Development of multifunctional nano/ultrafiltration membrane based on a chitosan thin film on alginate electrospun nanofibres. J. Clean. Prod. 2017, 156, 470–479. [Google Scholar] [CrossRef]
- Hasan, K.; Pervez, M.; Talukder, M.; Sultana, M.; Mahmud, S.; Meraz, M.; Bansal, V.; Genyang, C. A Novel Coloration of Polyester Fabric through Green Silver Nanoparticles (G-AgNPs@PET). Nanomaterials 2019, 9, 569. [Google Scholar] [CrossRef] [Green Version]
- Raza, Z.A.; Bilal, U.; Noreen, U.; Munim, S.A.; Riaz, S.; Abdullah, M.U.; Abid, S. Chitosan Mediated Formation and Impregnation of Silver Nanoparticles on Viscose Fabric in Single Bath for Antibacterial Performance. Fibers Polym. 2019, 20, 1360–1367. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Ali, S.W.; Joshi, M.; Rajendran, S.; Das, A.; Alagirusamy, R. Leveraging antibacterial efficacy of silver loaded chitosan nanoparticles on layer-by-layer self-assembled coated cotton fabric. Int. J. Biol. Macromol. 2020, 162, 548–560. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, P.; Zhang, Y.; Li, C. Durable Antibacterial and UV Protective Properties of Cotton Fabric Coated with Carboxymethyl Chitosan and Ag/TiO2 Composite Nanoparticles. Fibers Polym. 2021, 23, 386–395. [Google Scholar] [CrossRef]
- Tripathi, R.; Narayan, A.; Bramhecha, I.; Sheikh, J. Development of multifunctional linen fabric using chitosan film as a template for immobilization of in-situ generated CeO2 nanoparticles. Int. J. Biol. Macromol. 2019, 121, 1154–1159. [Google Scholar] [CrossRef]
- Botelho, C.M.; Fernandes, M.M.; Souza, J.M.; Dias, N.; Sousa, A.M.; Teixeira, J.A.; Fangueiro, R.; Zille, A. New Textile for Personal Protective Equipment—Plasma Chitosan/Silver Nanoparticles Nylon Fabric. Fibers 2021, 9, 3. [Google Scholar] [CrossRef]
- Hebeish, A.; Allam, E.; Abd El-Thalouth, I.; Ragheb, A.; Shahin, A.; Shaban, H. Multifunctional Smart Nanocolorants for Simultaneous Printing and Antibacterial Finishing of Cotton Fabrics. Egypt. J. Chem. 2018, 62, 621–637. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Zheng, W.; Duan, P.; Chen, J.; Zhang, Y.; Fu, F.; Diao, H.; Liu, X. One-pot fabrication of durable antibacterial cotton fabric coated with silver nanoparticles via carboxymethyl chitosan as a binder and stabilizer. Carbohydr. Polym. 2019, 204, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M. Chitosan-titanium oxide fibers supported zero-valent nanoparticles: Highly efficient and easily retrievable catalyst for the removal of organic pollutants. Sci. Rep. 2018, 8, 6260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzat, M.; Ghanim, M.; Nageh, H.; Hassanin, A.H.; Abdel-Moneim, A. Antimicrobial Activity of O-Carboxymethyl Chitosan Nanofibers Containing Silver Nanoparticles Synthesized by Green Method. J. Nano Res. 2016, 40, 136–145. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Ran, J.; He, M.; Li, W.; Cheng, D.; Wang, X. Growing ZnO Nanoparticles on Polydopamine-Templated Cotton Fabrics for Durable Antimicrobial Activity and UV Protection. Polymers 2018, 10, 495. [Google Scholar] [CrossRef] [Green Version]
- Stieberova, B.; Zilka, M.; Ticha, M.; Freiberg, F.; Caramazana-González, P.; McKechnie, J.; Lester, E. Application of ZnO Nanoparticles in a Self-cleaning Coating on a Metal Panel: An Assessment of Environmental Benefits. ACS Sustain. Chem. Eng. 2017, 5, 2493–2500. [Google Scholar] [CrossRef]
- Raghav, S.; Jain, P.; Kumar, D. Alginates: Properties and Applications. In Polysaccharides: Properties and Applications; Inamuddin, I., Ahamed, M.I., Boddula, R., Altalhi, T.A., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 399–421. [Google Scholar]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2015, 57, 1133–1152. [Google Scholar] [CrossRef]
- Ji, F.; Guo, X.; Liu, A.; Xu, P.; Tan, Y.; Wang, R.; Hao, L. In-situ synthesis of polypyrrole/silver for fabricating alginate fabrics with high conductivity, UV resistance and hydrophobicity. Carbohydr. Polym. 2021, 270, 118362. [Google Scholar] [CrossRef]
- Mahmud, S.; Sultana, M.; Pervez, M.; Habib, M.; Liu, H.-H. Surface Functionalization of “Rajshahi Silk” Using Green Silver Nanoparticles. Fibers 2017, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, S.; Pervez, N.; Taher, M.A.; Mohiuddin, K.; Liu, H.-H. Multifunctional organic cotton fabric based on silver nanoparticles green synthesized from sodium alginate. Text. Res. J. 2019, 90, 1224–1236. [Google Scholar] [CrossRef]
- Mahmud, S.; Pervez, M.N.; Hasan, K.M.F.; Taher, M.A.; Liu, H.-H. In situ synthesis of green AgNPs on ramie fabric with functional and catalytic properties. Emerg. Mater. Res. 2019, 8, 623–633. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, Y.; Yan, J.; Zhang, W. Fabrication of ZnO nanoparticle-coated calcium alginate nonwoven fabric by ion exchange method based on amino hyperbranched polymer. Mater. Lett. 2020, 270, 127624. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hassabo, A.G.; Mohamed, A.L.; Shaheen, T.I. Surface modification of SiO2 coated ZnO nanoparticles for multifunctional cotton fabrics. J. Colloid Interface Sci. 2017, 498, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Marković, D.; Tseng, H.-H.; Nunney, T.; Radoičić, M.; Ilic-Tomic, T.; Radetić, M. Novel antimicrobial nanocomposite based on polypropylene non-woven fabric, biopolymer alginate and copper oxides nanoparticles. Appl. Surf. Sci. 2020, 527, 146829. [Google Scholar] [CrossRef]
- Marković, D.; Radoičić, M.; Barudžija, T.; Radetić, M. Modification of PET and PA fabrics with alginate and copper oxides nanoparticles. Compos. Interfaces 2021, 28, 1171–1187. [Google Scholar] [CrossRef]
- Heliopoulos, N.S.; Kouzilos, G.N.; Giarmenitis, A.I.; Papageorgiou, S.K.; Stamatakis, K.; Katsaros, F.K. Viscose Fabric Functionalized with Copper and Copper Alginate Treatment Toward Antibacterial and UV Blocking Properties. Fibers Polym. 2020, 21, 1238–1250. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Li, X.; Xia, Y.; Wang, B.; Zhao, Z. Antibacterial activity and in vitro cytotoxicity evaluation of alginate-AgNP fibers. Text. Res. J. 2016, 87, 1377–1386. [Google Scholar] [CrossRef]
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Bastos Araruna, F.; Oliveira Sousa Araruna, F.; Lima Alves Pereira, L.P.; Aranha Brito, M.C.; Veras Quelemes, P.; de Araújo-Nobre, A.R.; de Oliveira, T.M.; da Silva, D.A.; de Souza de Almeida Leite, J.R.; Fernandes Coutinho, D.; et al. Green syntheses of silver nanoparticles using babassu mesocarp starch (Attalea speciosa Mart. ex Spreng.) and their antimicrobial applications. Environ. Nanotechnol. Monit. Manag. 2020, 13, 115260. [Google Scholar] [CrossRef]
- Goswami, B.; Mahanta, D. Starch and its Derivatives: Properties and Applications. In Polysaccharides: Properties and Applications; Inamuddin, I., Ahamed, M.I., Boddula, R., Altalhi, T.A., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 253–281. [Google Scholar]
- Maniglia, B.C.; Castanha, N.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E.D. Starch modification through environmentally friendly alternatives: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yao, Y.; Wang, Z.; Wu, H. Hydroxypropylation reduces gelatinization temperature of corn starch for textile sizing. Cellulose 2021, 28, 5123–5134. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Wu, L.; Liu, Q.; Cheng, X.; Xu, Z. Investigating the relationship between structure of itaconylated starch and its sizing properties: Viscosity stability, adhesion and film properties for wool warp sizing. Int. J. Biol. Macromol. 2021, 181, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Peychev, B.; Vasileva, P. Novel starch-mediated synthesis of Au/ZnO nanocrystals and their photocatalytic properties. Heliyon 2021, 7, e07402. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi Alashti, T.; Motakef-Kazemi, N.; Shojaosadati, S.A. In Situ Production and Deposition of Nanosized Zinc Oxide on Cotton Fabric. Iran. J. Chem. Chem. Eng. IJCCE 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Ultra, C.I.; Adora, N.M.; Dagalea, F.M.S.; Lim, K.M.R.C. Antimicrobial Activity of Nypa Fruticans (Nipa) Palm Starch and Zinc Oxide Nanoparticles (ZnONps) in Textile: Solving the Scarcity Using Local Resources. J. Pharm. Res. Int. 2021, 32, 84–89. [Google Scholar] [CrossRef]
- Raza, Z.A. In Situ Synthesis and Immobilization of Nanosilver on Knitted Cellulose Fabric. J. Nat. Fibers 2017, 15, 183–190. [Google Scholar] [CrossRef]
- Alagarasan, D.; Harikrishnan, A.; Surendiran, M.; Indira, K.; Khalifa, A.S.; Elesawy, B.H. Synthesis and characterization of CuO nanoparticles and evaluation of their bactericidal and fungicidal activities in cotton fabrics. Appl. Nanosci. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Saraf, P.; Abdollahi Movaghar, M.; Montazer, M.; Mahmoudi Rad, M. Bio and photoactive starch/MnO2 and starch/MnO2/cotton hydrogel nanocomposite. Int. J. Biol. Macromol. 2021, 193, 681–692. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.; Anbar, R.; Kodeh, F.S.; Elmanama, A. Preparation and antimicrobial activity of ZnO-NPs coated cotton/starch and their functionalized ZnO-Ag/cotton and Zn(II) curcumin/cotton materials. Sci. Rep. 2020, 10, 5410. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.A.-M.H.; Kishk, D.M.; Nada, A.A. Green and Durable Treatment for Multifunctional Cellulose-containing Woven Fabrics via TiO2-NP and HMTAP Processed in Semi-pilot Machine. Fibers Polym. 2021, 22, 2815–2825. [Google Scholar] [CrossRef]
- Amani, A.; Montazer, M.; Mahmoudirad, M. Low starch/corn silk/ZnO as environmentally friendly nanocomposites assembling on PET fabrics. Int. J. Biol. Macromol. 2021, 170, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Huh, S.-H.; Ullah, Z.; Pan, Y.-T.; Churchill, D.G.; Koo, B.H. LBL generated fire retardant nanocomposites on cotton fabric using cationized starch-clay-nanoparticles matrix. Carbohydr. Polym. 2021, 274, 118626. [Google Scholar] [CrossRef] [PubMed]
- Salimpour Abkenar, S.; Mohammad Ali Malek, R. Modification of Silk Yarn with β-Cyclodextrin Nanoparticles: Preparation, Characterization, and Natural Dyeing Properties. Starch-Stärke 2021, 73, 2000209. [Google Scholar] [CrossRef]
- Gonzalez Pereira, A.; Carpena, M.; García Oliveira, P.; Mejuto, J.C.; Prieto, M.A.; Simal Gandara, J. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, A.H.; Montazer, M.; Soleimani, N. In situ synthesis of polyamidoamine/β-cyclodextrin/silver nanocomposites on polyester fabric tailoring drug delivery and antimicrobial properties. React. Funct. Polym. 2020, 152, 104602. [Google Scholar] [CrossRef]
- Hedayati, N.; Montazer, M.; Mahmoudirad, M.; Toliyat, T. Cotton fabric incorporated with β-cyclodextrin/ketoconazole/Ag NPs generating outstanding antifungal and antibacterial performances. Cellulose 2021, 28, 8095–8113. [Google Scholar] [CrossRef]
- Sari, C.; Arik, B. The Comparison of the Effects of Β-Cyclodextrin Complex and Derivative Complex with Silver Nanoparticles on Cotton Fabric. Research Square. 2021. Available online: https://doi.org/10.21203/rs.3.rs-693021/v1 (accessed on 12 February 2022).
- Attarchi, N.; Montazer, M.; Toliyat, T.; Samadi, N.; Harifi, T. Novel cellulose fabric with multifunctional properties through diverse methods of Ag/TiO2/β-cyclodextrin nanocomposites synthesis. Cellulose 2017, 25, 1449–1462. [Google Scholar] [CrossRef]
- Mogrovejo-Valdivia, A.; Maton, M.; Garcia-Fernandez, M.J.; Tabary, N.; Chai, F.; Neut, C.; Martel, B.; Blanchemain, N. In Vitro Microbiological and Drug Release of Silver/Ibuprofen Loaded Wound Dressing Designed for the Treatment of Chronically Infected Painful Wounds. Antibiotics 2021, 10, 805. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Wang, Y.M.; Li, S.Z.; Feng, X.D.; Liu, L.H.; Wang, Y.; Zhao, L.P.; Zhang, X. Electrospinning and Catalytic Properties of Cyclodextrin Functionalized Polyoxymethylene (POM) Nanofibers Supported by Silver Nanoparticles. Adv. Polym. Technol. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef] [Green Version]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2019, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Saxena, I.M.; Brown, R.M. Cellulose Biosynthesis: Current Views and Evolving Concepts. Ann. Bot. 2005, 96, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lee, P.S. Development and applications of transparent conductive nanocellulose paper. Sci. Technol. Adv. Mater. 2017, 18, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Padrão, J.; Zille, A. Surface Chemistry of Nanocellulose and Its Composites. In Nanocellulose and Its Composites for Water Treatment Applications; Kumar, D., Ed.; CRC Press: Boca Raton, FL, USA, 2021; p. 230. [Google Scholar]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Yu, J.; Nierstrasz, V.A.; Bordes, R. Cellulose Nanofibril-Based Coatings of Woven Cotton Fabrics for Improved Inkjet Printing with a Potential in E-Textile Manufacturing. ACS Sustain. Chem. Eng. 2017, 5, 4793–4801. [Google Scholar] [CrossRef]
- Li, M.; Farooq, A.; Jiang, S.; Zhang, M.; Mussana, H.; Liu, L. Functionalization of cotton fabric with ZnO nanoparticles and cellulose nanofibrils for ultraviolet protection. Text. Res. J. 2021, 91, 2303–2314. [Google Scholar] [CrossRef]
- Gupta, D.; Gulrajani, M.L. Self cleaning finishes for textiles. In Functional Finishes for Textiles; Paul, R., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 257–281. [Google Scholar]
- Jiang, X.; Tian, X.; Gu, J.; Huang, D.; Yang, Y. Cotton fabric coated with nano TiO2-acrylate copolymer for photocatalytic self-cleaning by in-situ suspension polymerization. Appl. Surf. Sci. 2011, 257, 8451–8456. [Google Scholar] [CrossRef]
- Doganli, G.; Yuzer, B.; Aydin, I.; Gultekin, T.; Con, A.H.; Selcuk, H.; Palamutcu, S. Functionalization of cotton fabric with nanosized TiO2 coating for self-cleaning and antibacterial property enhancement. J. Coat. Technol. Res. 2015, 13, 257–265. [Google Scholar] [CrossRef]
- Kale, B.M.; Wiener, J.; Militky, J.; Rwawiire, S.; Mishra, R.; Jacob, K.I.; Wang, Y. Coating of cellulose-TiO2 nanoparticles on cotton fabric for durable photocatalytic self-cleaning and stiffness. Carbohydr. Polym. 2016, 150, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yunusov, K.H.E.; Mullajonova, S.V.; Sarymsakov, A.A.; Jalilov, J.Z.; Turakulov, F.M.; Rashidova, S.S.H.; Letfullin, R. Antibacterial effect of cotton fabric treated with silver nanoparticles of different sizes and shapes. Int. J. Nanomater. Nanotechnol. Nanomed. 2019, 5, 016–023. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Oshin, E.A.; Olawumi, A.M. Nanostructural computation of 4D printing carboxymethylcellulose (CMC) composite. Nano-Struct. Nano-Objects 2020, 21, 100423. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, S.-H.; Shalumon, K.T.; Chen, J.-P. Dual functional core–sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta Biomater. 2015, 26, 225–235. [Google Scholar] [CrossRef]
- Chen, C.-H.; Cheng, Y.-H.; Chen, S.-H.; Chuang, A.D.-C.; Chen, J.-P. Functional hyaluronic acid-polylactic acid/silver nanoparticles core-sheath nanofiber membranes for prevention of post-operative tendon adhesion. Int. J. Mol. Sci. 2021, 22, 8781. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef]
- Li, K.; Cui, S.; Hu, J.; Zhou, Y.; Liu, Y. Crosslinked pectin nanofibers with well-dispersed Ag nanoparticles: Preparation and characterization. Carbohydr. Polym. 2018, 199, 68–74. [Google Scholar] [CrossRef]
- Marques, M.S.; Zepon, Κ.M.; Heckler, J.M.; Morisso, F.D.P.; da Silva Paula, M.M.; Κanis, L.A. One-pot synthesis of gold nanoparticles embedded in polysaccharide-based hydrogel: Physical-chemical characterization and feasibility for large-scale production. Int. J. Biol. Macromol. 2019, 124, 838–845. [Google Scholar] [CrossRef]
- McCune, D.; Guo, X.; Shi, T.; Stealey, S.; Antrobus, R.; Kaltchev, M.; Chen, J.; Kumpaty, S.; Hua, X.; Ren, W.; et al. Electrospinning pectin-based nanofibers: A parametric and cross-linker study. Appl. Nanosci. 2018, 8, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan Methyl-Esterification and Plant Development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Alipour, R.; Khorshidi, A.; Shojaei, A.F.; Mashayekhi, F.; Moghaddam, M.J.M. Skin wound healing acceleration by Ag nanoparticles embedded in PVA/PVP/Pectin/Mafenide acetate composite nanofibers. Polym. Test. 2019, 79, 106022. [Google Scholar] [CrossRef]
- Akinalan Balik, B.; Argin, S.; Lagaron, J.M.; Torres-Giner, S. Preparation and Characterization of Electrospun Pectin-Based Films and Their Application in Sustainable Aroma Barrier Multilayer Packaging. Appl. Sci. 2019, 9, 5136. [Google Scholar] [CrossRef] [Green Version]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Price, R.D.; Berry, M.G.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, G.; Hilliou, L.; Bernardo, G.; Sousa-Pinto, I.; Adams, R.W.; Nilsson, M.; Villanueva, R.D. Tailoring kappa/iota-hybrid carrageenan from Mastocarpus stellatus with desired gel quality through pre-extraction alkali treatment. Food Hydrocoll. 2013, 31, 94–102. [Google Scholar] [CrossRef]
- Dionísio, M.; Grenha, A. Locust bean gum: Exploring its potential for biopharmaceutical applications. J. Pharm. Bioallied Sci. 2012, 4, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Eghbalifam, N.; Shojaosadati, S.A.; Hashemi-Najafabadi, S.; Khorasani, A.C. Synthesis and characterization of antimicrobial wound dressing material based on silver nanoparticles loaded gum Arabic nanofibers. Int. J. Biol. Macromol. 2020, 155, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.; Emam, H.; Fathalla, M.; Abdel-Aziz, M.; Zahran, M. Chemical synthesis of silver nanoparticles in its solid state: Highly efficient antimicrobial cotton fabrics for wound healing properties. Egypt. J. Chem. 2021, 64, 2697–2709. [Google Scholar] [CrossRef]
- Mohamed, A.L.; El-Naggar, M.E.; Shaheen, T.I.; Hassabo, A.G. Novel nano polymeric system containing biosynthesized core shell silver/silica nanoparticles for functionalization of cellulosic based material. Microsyst. Technol. 2016, 22, 979–992. [Google Scholar] [CrossRef]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms To Inform Redesign Strategies To Reduce Environmental Impact. Acc. Chem. Res. 2019, 52, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, F.; Wang, J.; Zu, Y.; Gu, Z.; Zhao, Y. A Safe-by-Design Strategy towards Safer Nanomaterials in Nanomedicines. Adv. Mater. 2019, 31, e1805391. [Google Scholar] [CrossRef] [PubMed]
- Saifi, M.A.; Khan, W.; Godugu, C. Cytotoxicity of Nanomaterials: Using Nanotoxicology to Address the Safety Concerns of Nanoparticles. Pharm. Nanotechnol. 2018, 6, 3–16. [Google Scholar] [CrossRef]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2020, 14, 166. [Google Scholar] [CrossRef]
- Schmutz, M.; Borges, O.; Jesus, S.; Borchard, G.; Perale, G.; Zinn, M.; Sips, Ä.A.J.A.M.; Soeteman-Hernandez, L.G.; Wick, P.; Som, C. A Methodological Safe-by-Design Approach for the Development of Nanomedicines. Front. Bioeng. Biotechnol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yu, T.; Yu, Z.; Hu, X.; Yin, D. Nanomaterials Safer-by-Design: An Environmental Safety Perspective. Adv. Mater. 2018, 30, e1705691. [Google Scholar] [CrossRef] [PubMed]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr. 2017, 38, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Długosz, O.; Szostak, K.; Staroń, A.; Pulit-Prociak, J.; Banach, M. Methods for Reducing the Toxicity of Metal and Metal Oxide NPs as Biomedicine. Materials 2020, 13, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, M.K.; Shim, Y.J.; Yoon, T.H. Effects of agglomeration on in vitro dosimetry and cellular association of silver nanoparticles. Environ. Sci. Nano 2018, 5, 446–455. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects Between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2022, in press. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.N.; Prasad, M. Metal/metal oxide nanoparticles: Toxicity concerns associated with their physical state and remediation for biomedical applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef]
- Sethi, S.; Saruchi; Medha; Thakur, S.; Kaith, B.S.; Sharma, N.; Ansar, S.; Pandey, S.; Kuma, V. Biopolymer starch-gelatin embedded with silver nanoparticle–based hydrogel composites for antibacterial application. Biomass Convers. Biorefinery 2022, in press. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Soltys, L.; Olkhovyy, O.; Tatarchuk, T.; Naushad, M. Green Synthesis of Metal and Metal Oxide Nanoparticles: Principles of Green Chemistry and Raw Materials. Magnetochemistry 2021, 7, 145. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on Nanoparticles and Nanostructured Materials: Bioimaging, Biosensing, Drug Delivery, Tissue Engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloid Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Shavandi, A.; Saeedi, P.; Ali, M.A.; Jalalvandi, E. Green synthesis of polysaccharide-based inorganic nanoparticles and biomedical aspects. In Functional Polysaccharides for Biomedical Applications; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 267–304. [Google Scholar]
- Worthington, K.L.S.; Adamcakova-Dodd, A.; Wongrakpanich, A.; Mudunkotuwa, I.A.; Mapuskar, K.A.; Joshi, V.B.; Allan Guymon, C.; Spitz, D.R.; Grassian, V.H.; Thorne, P.S.; et al. Chitosan coating of copper nanoparticles reducesin vitrotoxicity and increases inflammation in the lung. Nanotechnology 2013, 24, 395101. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Polysaccharide Function | NPs (Shape, Size) | Textile Substrate, Structure | Application | Results | Ref. |
|---|---|---|---|---|---|
| Antimicrobial activity | Ag (n.d. *) | Cotton, woven | Packaging | Antimicrobial activity against S. aureus, P. aeruginosa, C. albicans, and A. niger; chitosan increased air permeability and water absorbance | [47] |
| Ag (n.d.) | Cotton, woven | Medical and UV-protective textiles | Air and water permeability decreased, whereas tensile strength and elongation increased; superior UV blocking; antimicrobial activity against P. aeruginosa, S. aureus, A. niger, and C. albicans | [48] | |
| Ag (spherical, <100 nm) | Cotton, woven | Hygienic products | Antibacterial activity against S. aureus improved with the addition of AgNPs | [49] | |
| Chitosan-TiO2 and chitosan-TiO2/ZnO (spherical, 11.7 nm) | Cotton, woven | Antimicrobial, self-cleaning, and UV-protective textiles | Enhanced antibacterial activity against S. aureus, E. coli, and A. niger; improved self-cleaning and UV-protective properties | [50] | |
| Ag (n.d.) | PET, nonwoven | Antimicrobial textiles (wound dressings) | Improved antibacterial activity against E. coli and S. aureus | [51] | |
| Psidium guajava extract-ZnO (spherical, 12–18 nm and 5–7 nm (water and ethanol extract) | Cotton, woven | Antimicrobial textiles | Composite with ZnONPs had better antimicrobial activity and UV protection in the presence of chitosan | [52] | |
| PVP-Ag (n.d., 30 nm) | Acrylic acid and chitosan-grafted polypropylene, nonwoven | Antimicrobial textiles | Antibacterial resistance increased after coating with chitosan and improved further with the addition of AgNPs (E. coli, S. aureus, and B. subtilis) | [53] | |
| Antimicrobial activity; immobilization | Ag (n.d., 40–70 nm) | PET (n.d.) | Antimicrobial and flame-retardant textiles | Good antibacterial resistance against E. coli; flame retardance was improved with the addition of AgNPs along with chitosan | [54] |
| Chitosan-Ag (spherical, 20 nm) | Cotton, woven | Antibacterial, UV-protective, and flame-retardant textiles | Antimicrobial activity against E. coli, S. aureus, and C. albicans; small reduction after 20 washing cycles; improvement in UV-protective and flame-retardant properties | [55] | |
| CMCh-Ag (spherical, 10–20 nm) | Viscose, woven | Antimicrobial textiles (wound dressings) | Superior antibacterial activity against S. aureus compared to that against E. coli with increasing concentration of AgNPs | [56] | |
| Ag (n.d., 34.4 nm) | Cotton, woven | Antibacterial textiles | No cytotoxic effect on human skin; excellent antibacterial durability against E. coli and S. aureus achieved by a small Ag dosage | [57] | |
| Antimicrobial activity; immobilization; stabilizing agent | ZnO and TiO2 (rod-shaped, 18 nm) | Cotton, woven | Antimicrobial and UV-protective textiles | The durability of antibacterial efficiency against K. pneumonia and S. aureus increased up to 10 washing cycles the using sol–gel method | [58] |
| Antimicrobial activity; stabilizing agent | Chitosan-Cu (n.d., 20–30 nm) | Cotton and cotton/polyester, woven | Antimicrobial textiles | Antibacterial effect was predominantly observed against S. aureus in comparison with E. coli | [59] |
| Antimicrobial activity; substrate | Carboxymethyl pullulan-ZnO (spherical, 9 nm) | Cotton, woven | pH, thermo-sensitive, and antibacterial agents | Antimicrobial activity towards S. aureus and E. coli; textile sensitive to temperature between 24 and 40 °C and pH 3, 7, and 10 | [14] |
| Ag (n.d.) | Cotton, woven | Antimicrobial textiles | Improved antimicrobial properties against E. coli and B. subtilis | [60] | |
| Immobilization | Ginger oil-Ag (spherical, 14 nm) | Cotton, woven | Wound patches/gauzes | Gauzes with antimicrobial activity against C. albicans, E. coli, and S. aureus; improved UV protection; brilliant yellow-brownish color | [17] |
| Ag (n.d.) | Cotton, woven | Antimicrobial textiles, wound dressings | Good antibacterial activity against S. aureus and E. coli | [61] | |
| Tamarin-Ag (n.d., 20–50 nm) | Linen, woven | Antibacterial, UV-protective, and flame-retardant textiles | Antibacterial activity against S. aureus and E. coli; UV protection and improved antioxidant activity; moderate improvement of flame retardance | [62] | |
| Fe, Cu, Ag, Co, and Ni (n.d.) | Cotton, woven | Catalyst strips | High catalytic efficiency for the conversion of toxic substances from azo dyes and nitrophenols | [38] | |
| Co (n.d., 90 ± 22 nm) | Cotton, woven | Catalyst for the reduction of pollutants in water | CoNPs showed reduction of congo red dye (96% of the dye was degraded in only 21 min) and nitrophenols in aqueous solutions | [63] | |
| Cu (n.d., 80–90 nm) | Cotton, woven | Catalyst for dye reduction | Cu catalyst remained active even after three usages; excellent stability and recyclability during the degradation process | [64] | |
| ZnO and Ag (n.d., 35 and 40 nm) | Cotton, woven | Technical textiles with antimicrobial and UV protection properties | Antimicrobial action against S. aureus and E. coli; noticeable increase in UV blocking and in bending rigidity; functional properties maintained even after 15 washing cycles | [65] | |
| ZnO and TiO2 (n.d., 10–30 nm) and silicon dioxide (SiO2) (n.d., 10–20 nm) | Cotton/polyester, woven | Antibacterial and UV-protective textiles | Good antibacterial effect for fabrics coated with TiO2, followed by ZnO and SiO2; higher UPF for the samples with TiO2, followed by ZnO, SiO2NPs, and chitosan | [66] | |
| Fe (NO3)3 (n.d) | Ramie, woven | Flame-retardant textiles | Flame-retardant properties were improved; mechanical properties were reduced | [67] | |
| Reducing and stabilizing agent | Chitosan-Ag (spherical, n.d.) | Polyamide, woven | Antimicrobial textiles | Bacterial activity with the addition of AgNPs but reduced after 20 washing cycles; consistent color, even after one year | [68] |
| Chitosan-Ag (n.d.) | Sodium alginate, nanofibers | Antimicrobial textiles and filter for oil and dyes | Antibacterial effect on E. coli and S. aureus; rejection rate for oil and dye removal was significant and reduced after five filtration cycles | [69] | |
| Chitosan-Ag (n.d., 10–20 nm) | Polyester, woven | Coloration and antimicrobial textiles | Antibacterial activity improved but reduced after 10 washing cycles; improved color fastness | [70] | |
| Ag (spherical, 8.57 nm) | Viscose, woven | Antimicrobial textiles | Strong antibacterial activity against E. coli and S. aureus; tensile strength improved | [71] | |
| Reducing and stabilizing agent; immobilization | Chitosan-Ag (spherical, n.d.) | Aramid, woven | Coloration and antimicrobial activity | Improved thermal resistivity and color properties; excellent antibacterial action against E. coli and S. aureus, even after 10 washing cycles | [46] |
| Chitosan-Ag (multi-shape, 165 nm) | Cotton, woven | Antimicrobial textiles for biomedical applications | Antibacterial action against S. aureus and E. coli; coated fabric showed a higher release of Ag ions and for a longer time | [72] | |
| Stabilizing agent | CMCh-Ag/TiO2 (n.d.) | Cotton, woven | Antibacterial and UV-protective textiles | Antibacterial activity against E. coli and S. aureus; UPF 50+ | [73] |
| Chitosan-CeO2 (spherical, n.d.) | Linen, woven | Antibacterial, UV protective, flame-retardant, and easy-care textiles | Antibacterial activity against S. aureus and E. coli; flame retardance was improved with the coating of chitosan and furthermore improved with the addition of CeO2NPs; reduced efficacy after five washes; improved wrinkle resistance and UV protection | [74] | |
| Ag (n.d., 63.9–68.2 nm) | Cotton, woven | Antimicrobial textiles | Antibacterial activity against S. aureus and E. coli, even after more than 50 washing cycles | [13] | |
| PVA-Chitosan-PEG-Ag (n.d., 96 nm) | Cotton, woven | Antibacterial nasal tampons | Reduction in blood clotting time from 180 s to 90 s and antibacterial action against S. aureus and E. coli | [44] | |
| Chitosan-Ag (n.d., 25 nm) | Polyamide, woven | Antimicrobial textiles (masks) | AgNPs improved antibacterial activity against S. aureus and P. aeruginosa, but it was reduced to a greater extent after washing | [75] | |
| CuO, ZnO, TiO2, and Ag (n.d., 5.8, 11.9, 15.10, and 15.93 nm) | Cotton, woven | Antimicrobial textiles | AgNPs and CuONPs showed antibacterial activity against B. cereus and E. coli, whereas ZnONPs acted against Salmonella, B. cereus, and E. coli | [76] | |
| Stabilizing agent; immobilization | CMCh-Ag (spherical, 10–80 nm) | Cotton, woven | Antibacterial textiles | Improved antibacterial activity against E. coli and S. aureus before and after 50 washing cycles | [77] |
| Substrate | Glucose-Ag (spherical or polygon-like, n.d.) | Chitosan, non-woven | Conductive (electrocardiogram signals) and antimicrobial textiles | After eight washing cycles, the electrical resistance remained below 1 Ω·sq−1 | [16] |
| Co, Ni, Cu, and Ag (n.d., 26–33 nm) | Chitosan-TiO2 (<25 nm) nanofibers | Catalyst for theremoval of organic pollutants | High catalytic efficiency for the reduction of dyes and nitrophenols; good catalytic activity of Cu-composites | [78] | |
| CMCh-Ag/TiO2 (n.d., 5–15 nm) | PVA-chitosan, nanofibers | Antimicrobial textiles (wound dressings) | Antimicrobial activity against S. aureus, E. coli, K. pneumoniae, and C. albicans | [79] | |
| Substrate; stabilizing agent | Chitosan-PVA-ZnO (n.d., 40 nm) | Chitosan-PVA-ZnO, nanofibers | Scaffolds and diabetic wound dressings | Antibacterial properties against E. coli, P. aeruginosa, B. subtilis, and S. aureus; chitosan/PVA and chitosan/PVA/ZnO nanofiber membranes with higher antioxidant properties | [45] |
| Polysaccharide Function | NPs (Shape, Size) | Textile Substrate, Structure | Application | Results | Ref. |
|---|---|---|---|---|---|
| Immobilization | ZnO (n.d. *) | Cotton, woven | Antimicrobial and UV-protective textiles | Enhanced UPF values and antimicrobial activity against E. coli, S. aureus, and C. albicans | [52] |
| Reducing agent; substrate | Ag (n.d.) | Alginate, electrospun fibers | Sensors | Sensitive humidity sensor for breathing monitorization (humidity range between 20% and 85%) | [15] |
| Polypyrrole/Ag (n.d.) | Alginate, non-woven | Multifunctional textiles | Highly conductive, hydrophobic, and UV-resistant fabric; antistatic properties improved; thermally stable | [85] | |
| Reducing and stabilizing agent | Ag (n.d., 6–10 nm) | Silk, woven | Multifunctional textiles | Fabric coloration; improved light and washing fastness and mechanical properties; antibacterial activity against E. coli and S. aureus; UV protection | [86] |
| Ag (n.d., 8.2 nm) | Organic cotton, woven | Multifunctional textiles | Fabric coloration; washing fastness improvement; antibacterial activity against E. coli and S. aureus; UV protection | [87] | |
| Ag (n.d.) | Ramie, fiber | Multifunctional fibers | Fabric coloration; improved mechanical properties; antibacterial activity against E. coli and S. aureus; UV protection; reductor of 4-nitrophenol | [88] | |
| Reducing and stabilizing agent; substrate | ZnO (rice-shaped, 100 nm) | Calcium alginate, non-woven | n.d. | Facile fabrication of ZnONPs by in situ synthesis on calcium alginate fabric | [89] |
| Stabilizing agent | Ag (n.d.) | Cotton gauze, non-woven | Antimicrobial textiles (wound dressing) | Excellent antibacterial efficiency against E. coli and S. aureus; improved water absorbency, water holding capacity, and vertical wicking | [61] |
| SiO2/ZnO (spherical, 203.7 nm) | Cotton, woven | Antibacterial and UV-protective textiles | Antibacterial activity against E. coli and S. aureus; UPF 50+ | [90] | |
| CuO and Cu2O (n.d., 45 and 43 nm, respectively) | Polypropylene, non-woven | Antimicrobial textiles | Excellent antimicrobial activity against E. coli, S. aureus, and C. albicans; non-cytotoxic to HaCaT cells | [91] | |
| CuO and Cu2O (n.d., 16–90 nm) | Polyester and polyamide, woven | Antimicrobial textiles | Excellent antimicrobial activity against E. coli, S. aureus, and C. albicans on polyester; good antimicrobial activity on polyamide | [92] | |
| Stabilizing agent; immobilization | CuO (n.d.) | Viscose, woven | Antibacterial and UV-protective textiles | Excellent antibacterial activity against cyanobacterium Synechocystis sp.; improved UPF, washing fastness, and mechanical properties | [93] |
| Substrate | Ag (spherical, 10–25 nm) | Alginate, wet-spun fibers | Antibacterial textiles | Excellent antibacterial activity against E. coli and S. aureus; cytotoxic effects against cancer HeLa cells | [94] |
| Ag (n.d.) | Chitosan/PET/alginate, LBL composite | Nano/ultrafiltration membranes | Antibacterial activity against E. coli and S. aureus; remotion of oils up to 93%; NP retention greater than 98% | [69] |
| Polysaccharide Function | NPs (Shape, Size) | Textile Substrate, Structure | Application | Results | Ref. |
|---|---|---|---|---|---|
| Immobilization | ZnO (flakes and nanoflowers, 16.2 nm) | Cotton, woven | Antibacterial textile | ZnO/cotton–starch (3%) with bacterial reduction of 96% (S. aureus) and 76% (E. coli) | [108] |
| ZnO (spherical, 52.42 nm); ZnO on fabric (hexagonal, 11.96 nm) | Polyester, woven | Multifunctional textiles (flame-retardant, self-cleaning, antimicrobial) | Flame-retardant with no dripping; hydrophobic with self-cleaning properties (∆RGB of 73.9); cell viability of 129%; bacteria reduction of 97%, 100%, and 94% (E. coli, S. aureus, and C. albicans, respectively) | [110] | |
| TiO2 (n.d. *, 200 nm) | Cotton, woven | Flame retardant | Seven bilayers: pyrolysis reduction of 30%; peak heat release rate (PHRR) of 193 W·g−1; Limiting oxygen index (LOI) of 22.2% | [111] | |
| TiO2 (n.d., 50–100 nm) | Cotton, linen, viscose, polyester, and their blends, woven | Multifunctional textiles (antimicrobial, self-cleaning, UV-protective) | Bacterial reduction of 85% (S. aureus); self-cleaning of 91%; UPF of 277 (cotton) | [109] | |
| Reducing agent | CuO (spherical, 10–100 nm) | Cotton, woven | Antimicrobial textiles (medical, cosmetic, sports) | Hydrophobicity (WCA of 110°); antimicrobial activity of 96%, 94%, 92%, and 89% (against S. aureus, E. coli, P. fuorescens, B. subtilis, and C. albicans, respectively); washing durability | [106] |
| MnO2 (n.d.) | Cotton, woven | Agriculture, medical textile, water treatment | Superabsorbent (227%); photocatalytic (∆RGB of 75); good antimicrobial properties for the hydrogel but very low for the fabric treated with the hydrogel (poor adhesion) | [107] | |
| Reducing and stabilizing agent | Ag (n.d., 25.7 nm) | Cotton, knit | Medical textiles, water purification | Antibacterial activity against S. aureus and E. coli (halo) | [105] |
| Stabilizing agent | ZnO (spherical, 88 nm) | Cotton, woven | Antibacterial textiles | Hydrophobicity (WCA of 95.5°); antimicrobial activity with a zone of inhibition of 1 mm (E. coli); washing durability | [103] |
| ZnO (n.d.) | Face masks, non-woven | Face masks | Antimicrobial activity of the ZnONPs with a zone of inhibition of 3.67 and 2.33 mm (S. aureus and E. coli, respectively) | [104] |
| Polysaccharide Function | NPs (Shape, Size) | Textile Substrate, Structure | Application | Results | Ref. |
|---|---|---|---|---|---|
| Reducing agent; immobilization | Ag/TiO2/β-CDs (semi-spherical, 48 nm) | Cotton, woven | Antibacterial textile, self-cleaning, environmental remediation | Ag/TiO2/β-CDs samples with excellent self-cleaning properties (methylene blue); antibacterial activity against S. aureus of 96.8% | [117] |
| Ag (n.d. *) | PET, non-woven | Wound dressing, antibacterial, drug release | Poly-CDs: Ag adsorption of 450 μg·cm−2 (24 h), Ag release of 23 μg·cm−2 (3 days), bacterial reduction of 4 log10 (S. aureus) and 6 log10 (E. coli); PEM coating: reduced Ag diffusion (8.0 μg·cm−2), bacterial reduction of 3 log10 (S. aureus) and 5 log10 (E. coli) | [51] | |
| Ag (n.d.) | PET, non-woven | Wound dressing, antibacterial, and antalgic drug release | PEM system allowed for complete IBU-L release in 6 h; PET-CD-Ag-PEM had a bacterial reduction of 4 log10 against S. aureus and E. coli; cell viability of 0% | [118] | |
| Reducing and stabilizing agent | β-CDs/Ag (2%) (n.d., 272.6 nm); β-CDs/KZ/Ag (2%) (n.d., 904.0 nm) | Cotton, woven | Medical applications, wound dressings, sportswear for sensitive skin | β-CD/Ag (2%): microbial reduction of 70, 42, 87, and 82% (C. albicans, A. niger, E. coli, and S. aureus, respectively); β-CD/KZ/Ag (2%): microbial reduction of 100% in C. albicans and A. niger and about 85% in E. coli and S. aureus; good washing durability (30 washing cycles) | [115] |
| Stabilizing agent; immobilization | Ag2O (n.d., 20.6 nm); Ag/β-CDs (n.d., 9.5 nm) | Polyester, woven | Drug release and antimicrobial textile | Drug release of 45% (150 h); microbial reduction in E. coli, S. aureus, and C. albicans of 100%, 100%, and 99%, respectively | [114] |
| Ag (cubic, 31 nm) | Cotton, woven | Antibacterial textile | S-β-CDs + AgNPs + EDTA with a bacterial reduction in S. aureus of 95% and 79% and in E. coli of 95% and 77% (before and after 10 washing cycles, respectively) | [116] | |
| Ag (n.d.) | POM/β-CD electrospun microfiber mat | Waste treatment, molecular recognition, catalysis | Ag/POM/β-CDs mats (average fiber diameter of 6.4 μm) with excellent catalytic degradation of organic dyes in the presence of NaBH4 | [119] |
| Polysaccharide Function (Cellulose Type) | NPs (Shape, Size) | Textile Substrate, Structure | Application | Results | Ref. |
|---|---|---|---|---|---|
| Immobilization (CNFs) | Ag-NH2 (spherical, ~20 nm) | CNFs and gelatin, non-woven | Wound dressing | Improved mechanical, self-recovery, and hemostatic (gelation) properties; antibacterial properties against S. aureus and P. aeruginosa; fluid balance on the wound bed | [127] |
| Ag (n.d. *) | Cotton, woven | Disposable e-textiles (electronic devices integrated into fabrics) | Better surface wetting and improved inkjet printing process; higher-speed inkjet printing | [128] | |
| ZnO (n.d., 90 ± 10 nm) | Cotton, woven | UV-protective textiles | Reduced the agglomeration of ZnO; decreased air permeability; improved mechanical properties; showed a bacteriostatic inhibition effect against E. coli and S. aureus | [129] | |
| Immobilization (viscose) | TiO2 (n.d., 50 nm) | Cotton | n.d. | Photocatalytic self-cleaning and permanently stiff cotton properties; increased degradation rate of orange II dye under UV–vis light irradiation | [133] |
| Reducing and stabilizing agent (Na-CMC) | Ag (spherical, 2–8 nm, 5–35 nm; whiskers, L: 130–420 nm, W: 15–40 nm) | Cotton, woven | Antibacterial textiles | Bactericidal activity against bacterium S. epidermidis and fungus C. albicans | [134] |
| Polysaccharide Function | NPs (Shape, Size) | Textile Substrate, Structure | Application | Results | Ref. |
|---|---|---|---|---|---|
| Antimicrobial activity (Dextran) | Ag (spherical, 8–58 nm) | Cotton, n.d. * | Wound dressing | Formulations exhibited moderate antimicrobial activity against A. niger, C. albicans, S. aureus, and E. coli | [150] |
| Reducing and stabilizing agent (κ-carrageenan and locust bean gum) | Au (spherical, 21–45 nm) | n.d. | General use | κ-carrageenan and locust bean gum reduced and stabilized AuNPs; the formulation can be laminated on non-woven fabric at industrial large scale | [140] |
| Stabilizing agent (pectin) | Ag (n.d. *, 24 nm) | Pectin, PVA, PVP, and mafenide acetate, non-woven | Wound healing | Low antibacterial activity against S. aureus, E. coli, and P. aeruginosa; acceptable cytotoxicity, including faster in vivo wound healing | [143] |
| Stabilizing agent (pullulan) | Ag (spherical, 20 nm; in sodium silicate) | Cotton, n.d. | n.d. | Functionalized cotton water uptake became stimuli-responsive to pH and temperature between 24 and 30 °C (neutral and acid pH) | [151] |
| Substrate (pectin and hyaluronic acid) | Ag (spherical, 8.6 nm) | Pectin, hyaluronic acid, and PVA, non-woven | Wound dressing | High antimicrobial activity against B. subtilis, S. aureus, and E. coli; histological analysis displayed a faster healing process, attributed to the presence of hyaluronic acid | [138] |
| Substrate (pectin) | Ag (spherical, 3.7–8.6 nm) | Pectin, non-woven | Wound healing, catalysis, and Raman enhancement | AgNPs homogeneously distributed in the pectin nanofibers, and their size may be tailored; AgNP release took 4 weeks | [139] |
| Substrate (polycaprolactone and hyaluronic acid) | Ag (spherical, 4–10 nm) | Polycaprolactone and hyaluronic acid, non-woven | Prevention of post-operative tendon adhesion | Nanofiber sheath of polycaprolactone as tendon-sheet surrogate; core contains hyaluronic acid to prevent cell adhesion and AgNPs as antimicrobial agent; suitable cytotoxicity; low antimicrobial activity against S. aureus and E. coli; histological observations revealed promising antiadhesive properties | [136] |
| Substrate (polylactic acid and hyaluronic acid) | Ag (spherical and rods, 10–40 nm) | Polylactic acid, hyaluronic acid, non-woven | Prevention of post-operative tendon adhesion | Polylactic acid worked as a tendon-sheet surrogate, hyaluronic acid prevented cell adhesion, and AgNPs were responsible for the antimicrobial effect; most tested formulations exhibited acceptable cytotoxicity (>70%); weak antimicrobial activity against S. aureus and E. coli; in vivo tests with rats showed no blood, renal, or liver problems; histological observation denoted low adhesion in some formulations | [137] |
| Substrate (PVA, gum arabic, and polycaprolactone) | Ag (spherical, 10–100 nm) | PVA, gum arabic, and polycaprolactone, non-woven | Wound dressing | Low antimicrobial activity against S. aureus, E. coli, P. aeruginosa, and C. albicans. Improved adequacy of water-vapor permeability and porosity for wound-dressing use; suitable cytotoxicity | [149] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, M.; Padrão, J.; Ribeiro, A.I.; Fernandes, R.D.V.; Melro, L.; Nicolau, T.; Mehravani, B.; Alves, C.; Rodrigues, R.; Zille, A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials 2022, 12, 1006. https://doi.org/10.3390/nano12061006
Fernandes M, Padrão J, Ribeiro AI, Fernandes RDV, Melro L, Nicolau T, Mehravani B, Alves C, Rodrigues R, Zille A. Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review. Nanomaterials. 2022; 12(6):1006. https://doi.org/10.3390/nano12061006
Chicago/Turabian StyleFernandes, Marta, Jorge Padrão, Ana I. Ribeiro, Rui D. V. Fernandes, Liliana Melro, Talita Nicolau, Behnaz Mehravani, Cátia Alves, Rui Rodrigues, and Andrea Zille. 2022. "Polysaccharides and Metal Nanoparticles for Functional Textiles: A Review" Nanomaterials 12, no. 6: 1006. https://doi.org/10.3390/nano12061006