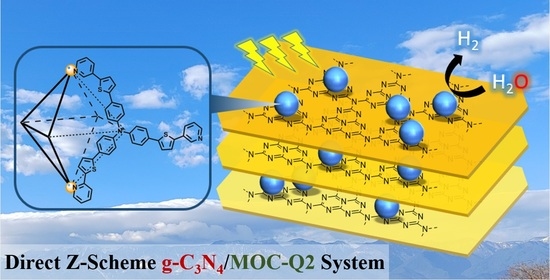
Direct Z-Scheme Heterojunction Catalysts Constructed by Graphitic-C3N4 and Photosensitive Metal-Organic Cages for Efficient Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Synthesis and Preparation
2.3.1. Synthesis of MOC-Q2
2.3.2. Preparation of g-C3N4
2.3.3. Preparation of g-C3N4/MOC-Q2
2.3.4. Preparation of g-C3N4/L-2 (0.7 wt%)
2.3.5. Preparation of Pd/g-C3N4/L-2 (0.7 wt%)
2.4. Photocatalytic H2 Generation
2.5. Apparent Quantum Yield (AQY) Measurements for H2 Evolution
2.6. Hydroxyl Radical Trapping Experiment
3. Results and Discussion
3.1. Synthesis of MOC-Q2 and g-C3N4/MOC-Q2
3.2. Characterization of g-C3N4/MOC-Q2
3.3. Photocatalytic H2 Evolution
3.4. Mechanism Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the Art and Perspectives in Heterogeneous Catalysis of CO2 Hydrogenation to Methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, Y.; Kawano, M.; Fujita, M. Crystalline Molecular Flasks. Nat. Chem. 2011, 3, 349–358. [Google Scholar] [CrossRef]
- Zarra, S.; Wood, D.M.; Roberts, D.A.; Nitschke, J.R. Molecular Containers in Complex Chemical Systems. Chem. Soc. Rev. 2015, 44, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Hardy, M.; Lutzen, A. Better Together: Functional Heterobimetallic Macrocyclic and Cage-like Assemblies. Chem. Eur. J. 2020, 26, 13332–13346. [Google Scholar] [CrossRef]
- Pilgrim, B.S.; Champness, N.R. Metal-Organic Frameworks and Metal-Organic Cages—A Perspective. Chempluschem 2020, 85, 1842–1856. [Google Scholar] [CrossRef]
- Pan, M.; Wu, K.; Zhang, J.-H.; Su, C.-Y. Chiral Metal-Organic Cages/Containers (MOCs): From Structural and Stereochemical Design to Applications. Coord. Chem. Rev. 2019, 378, 333–349. [Google Scholar] [CrossRef]
- Wu, K.; Li, K.; Chen, S.; Hou, Y.-J.; Lu, Y.-L.; Wang, J.-S.; Wei, M.-J.; Pan, M.; Su, C.-Y. The Redox Coupling Effect in a Photocatalytic RuII-PdII Cage with TTF Guest as Electron Relay Mediator for Visible-Light Hydrogen-Evolving Promotion. Angew. Chem. Int. Ed. Engl. 2020, 59, 2639–2643. [Google Scholar] [CrossRef]
- Chen, S.; Li, K.; Zhao, F.; Zhang, L.; Pan, M.; Fan, Y.-Z.; Guo, J.; Shi, J.-Y.; Su, C.-Y. A Metal-Organic Cage incorporating Multiple Light Harvesting and Catalytic Centres for Photochemical Hydrogen Production. Nat. Commun. 2016, 7, 13169. [Google Scholar] [CrossRef] [Green Version]
- Manbeck, G.F.; Fujita, E.; Brewer, K.J. Tetra- and Heptametallic Ru(II), Rh(III) Supramolecular Hydrogen Production Photocatalysts. J. Am. Chem. Soc. 2017, 139, 7843–7854. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, Q.; Zhang, Y.; Duan, C. Electron Transfer in the Confined Environments of Metal-Organic Coordination Supramolecular Systems. Chem. Soc. Rev. 2020, 49, 5561–5600. [Google Scholar] [CrossRef] [PubMed]
- Mollick, S.; Fajal, S.; Mukherjee, S.; Ghosh, S.K. Stabilizing Metal-Organic Polyhedra (MOP): Issues and Strategies. Chem. Asian J. 2019, 14, 3096–3108. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-C.; Chu, K.-L.; Shi, J.-Y.; Wu, D.-J.; Wang, X.-D.; Mayor, M.; Su, C.-Y. Heterogenization of Photochemical Molecular Devices: Embedding a Metal−Organic Cage into a ZIF-8-Derived Matrix to Promote Proton and Electron Transfer. J. Am. Chem. Soc. 2019, 141, 13057–13065. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Ma, Z.; Dong, L.; Jia, X.; Zhang, J. Enhancing Photocatalytic Hydrogen Production of g-C3N4 by Selective Deposition of Pt Cocatalyst. Nanomaterials 2021, 11, 3266. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, A.; Hou, J.; Liu, Q.; Li, H.; Guo, X. Ag-Au Core-Shell Triangular Nanoprisms for Improving p-g-C3N4 Photocatalytic Hydrogen Production. Nanomaterials 2021, 11, 3347. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Nguyen, T.K.A.; Dao, D.Q.; Shin, E.W. Ethanol Solvothermal Treatment on Graphitic Carbon Nitride Materials for Enhancing Photocatalytic Hydrogen Evolution Performance. Nanomaterials 2022, 12, 179. [Google Scholar] [CrossRef]
- Ge, F.; Huang, S.; Yan, J.; Jing, L.; Chen, F.; Xie, M.; Xu, Y.; Xu, H.; Li, H. Sulfur Promoted n-π* Electron Transitions in Thiophene-Doped g-C3N4 for Enhanced Photocatalytic Activity. Chin. J. Catal. 2021, 42, 450–459. [Google Scholar] [CrossRef]
- Bai, L.; Huang, H.; Zhang, S.; Hao, L.; Zhang, Z.; Li, H.; Sun, L.; Guo, L.; Huang, H.; Zhang, Y. Photocatalysis-Assisted Co3O4/g-C3N4 p–n Junction All-Solid-State Supercapacitors: A Bridge between Energy Storage and Photocatalysis. Adv. Sci. 2020, 7, 2001939. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.; Bahadur, I.; Karlapudi, S.; Jiang, Y.J. A Latest Overview on Photocatalytic Application of g-C3N4 Based Nanostructured Materials for Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 337–379. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhang, H.; Xiang, Q. Porous Graphitic Carbon Nitride for Solar Photocatalytic Applications. Nanoscale Horiz. 2020, 5, 765–786. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, H.; Li, X.; Fan, J.; Xiang, Q. Carbon-Graphitic Carbon Nitride Hybrids for Heterogeneous Photocatalysis. Small 2021, 17, 2005231. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial Photosynthesis for Solar Water-Splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Cui, J.; Li, X.; Zhang, Y.; Wang, C.; Yu, X.; Ye, J. Defective g-C3N4/Covalent Organic Framework Van Der Waals Heterojunction toward Highly Efficient S-scheme CO2 Photoreduction. Appl. Catal. B-Environ. 2022, 301, 120814. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Chai, Y.; Zhang, Z.; Zhu, Y. Efficient Photocatalytic Overall Water Splitting Induced by the Giant Internal Electric Field of a g-C3N4/rGO/PDIP Z-Scheme Heterojunction. Adv. Mater. 2021, 33, 2007479. [Google Scholar] [CrossRef]
- Hou, C.-P.; Chen, X.-L.; Huang, Z.-J.; Lei, Y.; Xiao, L.-M.; Huang, J.-F.; Li, S.-Y.; Liu, J.-M. Robust Heterogeneous Photocatalyst for Visible-Light-Driven Hydrogen Evolution Promotion: Immobilization of a Fluorescein Dye-Encapsulated Metal-Organic Cage on TiO2. ACS Appl. Mater. Interfaces 2021, 13, 57230–57240. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Liu, L.; Wu, D.-J.; Guo, J.; Shi, J.-Y.; Liu, J.-M.; Su, C.-Y. Immobilization of Metal-Organic Molecular Cage on g-C3N4 Semiconductor for Enhancement of Photocatalytic H2 Generation. Chin. J. Catal. 2019, 40, 1198–1204. [Google Scholar] [CrossRef]
- Qin, S.; Lei, Y.; Guo, J.; Huang, J.-F.; Hou, C.-P.; Liu, J.-M. Constructing Heterogeneous Direct Z-Scheme Photocatalysts Based on Metal−Organic Cages and Graphitic-C3N4 for High-Efficiency Photocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 25960–25971. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Lei, Y.; Huang, J.-F.; Xiao, L.-M.; Liu, J.-M. A Robust Photocatalytic Hybrid Material Composed of Metal Organic Cages and TiO2 for Efficient Visible-Light-Driven Hydrogen Evolution. Chem. Asian J. 2021, 16, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hoffman, M.Z. Reductive Quenching of the Excited States of Ruthenium(II) Complexes Containing 2,2′-Bipyridine, 2,2′-Bipyrazine, and 2,2′-Bipyrimidine Ligands. J. Phys. Chem. A 1994, 98, 11719–11726. [Google Scholar] [CrossRef]
- Ji, C.; Wang, W.; El-Sayed, E.; Liu, G.; Si, Y.; Su, K.; Ju, Z.; Wu, F.; Yuan, D. A High-Efficiency Dye-Sensitized Pt(II) Decorated Metal-Organic Cage for Visible-Light-Driven Hydrogen Production. Appl. Catal. B-Environ. 2021, 285, 119782. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, M.; Lu, Y.; Wei, Z.; Wang, H.; Pan, M. Ultrafine Palladium Nanoparticles Stabilized in the Porous Liquid of Covalent Organic Cages for Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2020, 3, 12108–12114. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Q.; Qin, C.; Sun, C.; Wang, X.; Su, Z. An Amine-Functionalized Zirconium Metal–Organic Polyhedron Photocatalyst with High Visible-Light Activity for Hydrogen Production. Chem. Eur. J. 2019, 25, 2824–2830. [Google Scholar] [CrossRef]
- Kumar, K.; Mane, V.; Yadav, A.; Kumbhar, A.; Boomishankar, R. Photochemical hydrogen evolution from water by a 1D-network of octahedral Ni6L8 cages. Chem. Commun. 2019, 55, 13156–13159. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, L.; Wei, J.; He, C.; Long, S.; Duan, C. Negatively Charged Metal–Organic Hosts with Cobalt Dithiolene Species: Improving PET Processes for Light-Driven Proton Reduction through Host-Guest Electrostatic Interactions. Chem. Commun. 2019, 55, 8524–8527. [Google Scholar] [CrossRef]
- Nurttila, S.; Becker, R.; Hessels, J.; Woutersen, S.; Reek, J. Photocatalytic Hydrogen Evolution by a Synthetic [FeFe] Hydrogenase Mimic Encapsulated in a Porphyrin Cage. Chem. Eur. J. 2018, 24, 16395–16406. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wei, J.; Zhang, J.; He, C.; Duan, C. Encapsulation of a Quinhydrone Cofactor in the Inner Pocket of Cobalt Triangular Prisms: Combined Light-Driven Reduction of Protons and Hydrogenation of Nitrobenzene. Angew. Chem. Int. Ed. 2017, 56, 15284–15288. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Wu, P.; He, C.; Guo, X.; Duan, C. DHPA-Containing Cobalt-Based Redox Metal-Organic Cyclohelicates as Enzymatic Molecular Flasks for Light-Driven H2 Production. Sci. Rep. 2017, 7, 14347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Wei, J.; Zhang, F.; He, C.; Zheng, S.; Duan, C. Redox-Active Copper Triangles as an Enzymatic Molecular Flask for Light-Driven Hydrogen Production. RSC Adv. 2017, 7, 48989–48993. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Yang, Y.; He, C.; Chang, Z.; Reek, J.; Duan, C. Control of Redox Events by Dye Encapsulation Applied to Light-Driven Splitting of Hydrogen Sulfide. Angew. Chem. Int. Ed. 2017, 56, 11759–11763. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.; Mane, V.; Kumbhar, A.; Boomishankar, R. Light-driven Hydrogen Evolution from Water by a Tripodal Silane Based CoII6L18 Octahedral Cage. Inorg. Chem. 2017, 56, 13286–13292. [Google Scholar] [CrossRef]
- Yu, H.; He, C.; Xu, J.; Duan, C.; Reek, J. Metal—Organic Redox Vehicles to Encapsulate Organic Dyes for Photocatalytic Protons and Carbon Dioxide Reduction. Inorg. Chem. Front. 2016, 3, 1256–1263. [Google Scholar] [CrossRef]
- Yang, L.; He, C.; Liu, X.; Zhang, J.; Sun, H.; Guo, H. Supramolecular Photoinduced Electron Transfer between a Redox-Active Hexanuclear Metal-Organic Cylinder and an Encapsulated Ruthenium(II) Complex. Chem. Eur. J. 2016, 22, 5253–5260. [Google Scholar] [CrossRef]
- Yang, L.; Jing, X.; He, C.; Chang, Z.; Duan, C. Redox-Active M8L6 Cubic Hosts with Tetraphenylethylene Faces Encapsulate Organic Dyes for Light-Driven H2 Production. Chem. Eur. J. 2016, 22, 18107–18114. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Yang, Y.; Duan, C. A Metal-Organic Tetrahedron as a Redox Vehicle to Encapsulate Organic Dyes for Photocatalytic Proton Reduction. J. Am. Chem. Soc. 2015, 137, 3967–3974. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, J.; Fu, X.; Wang, X. Metal-Free Photocatalytic Degradation of 4-Chlorophenol in Water by Mesoporous Carbon Nitride Semiconductors. Catal. Sci. Technol. 2012, 2, 1396–1402. [Google Scholar] [CrossRef]










| No. | Reference | Photocatalyst | H2 Yield | TON | Time |
|---|---|---|---|---|---|
| 1 | Appl. Catal. B-Environ. 2021, 285, 119782. [34] | RhB-Zr-Bpydc-PtCl2 MOCs | 230 μmol/g | 378[Pt] | 22 h |
| 2 | ACS Appl. Mater. Interfaces 2021, 13, 25960. (our work) [31] | g-C3N4/MOC-Q1 | 83,692 μmol/g | 19,268[MOC-Q1] | 25 h |
| 3 | ACS Appl. Mater. Interfaces 2021, 13, 57230. (our work) [29] | FL@MOC-PC6-TiO2 | 29.8 mmol/g | 4356[MOC-PC-6] | 40 h |
| 4 | Chem Asian J. 2021, 16, 2055.(our work) [32] | TiO2-MOC-Q2 | 309.8 mmol/g | 11,739[MOC-Q2] | 35 h |
| 5 | ACS Appl. Energy Mater. 2020, 3, 12108. [35] | PdNPs@C4R/g-C3N4 | 5487 μmol/g h | – | 20 h |
| 6 | Chem. Eur. J. 2019, 25,2824. [36] | Pt/ZrT-1-NH2 | 1060 μmol/g h | – | 4 h |
| 7 | Angew. Chem. Int. Ed. 2019, 59, 2639. [9] | MOC-16/TTF | 3173 μmol/μM | 1202[Pd] | 47 h |
| 8 | J. Am. Chem. Soc. 2019, 141, 13057. [14] | MOC-16@CZIF | 97.0 mmol/g | 35,000[Pd] | 24 h |
| 9 | Chem. Commun. 2019, 55, 13156. [37] | [Ni6L8]∞ | 14 μmol/μM | 2824[[Ni6L8]∞ | 69 h |
| 10 | Chinese J. Catal. 2019, 40, 1198.(our work) [30] | MOC-16/g-C3N4 | 10.0 mmol/g | 517[Pd] | 15 h |
| 11 | Chem. Commun. 2019, 55, 8524. [38] | Co-NAS/Ru(bpy)32+ | 10 mL/mM | 360[Co-NAS] | 9 h |
| 12 | Chem. Eur. J. 2018, 24, 16395. [39] | 1·Fe4(Zn-L)6 | – | 0.4[1·Fe4(Zn-L)6] | 2 h |
| 13 | Angew. Chem. Int. Ed. 2017, 56, 15284. [40] | Co-TPC/QHQ | 0.4 mL/μM | – | 12 h |
| 14 | Sci. Rep. 2017, 7, 14347. [41] | Co-ZPB/Fl | 50 mL/mM | 400[Co-ZPB] | 4 h |
| 15 | RSC Adv. 2017, 7, 48989. [42] | Cu-OBP/Fl | 0.1 mL/μM | 1200[Cu-OBP] | 20 h |
| 16 | Angew. Chem. Int. Ed. 2017, 56, 11759. [43] | Ni-TFT/Fl | 0.3 mL/mM | 25,000[Ni-TFT] | 20 h |
| 17 | Inorg. Chem. 2017, 56, 13286. [44] | Co6L8/Ru(bpy)3 | 12 μmol/h | 43[Co6L8] | 2 h |
| 18 | Inorg. Chem. Front. 2016, 3, 1256. [45] | Ni-SSC/Fl | 1 mL/μM | 1250[Ni-SSC] | 8 h |
| 19 | Chem. Eur. J. 2016, 22,5253. [46] | Ni-YL/Ru(dcbpy)3 | 0.2 mL/μM | 1600[Ni-YL] | 5 h |
| 20 | Chem. Eur. J. 2016, 22,18107. [47] | Cage2/Fl | 0.1 mL/μM | 700[Cage2] | 15 h |
| 21 | Nat. Commun. 2016, 7, 13169. [10] | MOC-16 | 50 μmol/μM | 635[Pd] | 48 h |
| 22 | J. Am. Chem. Soc. 2015, 137, 3967. [48] | Co-TFT/Fl | 1.5 mL/μM | 11,000[Co-TFT] | 15 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Qin, S.; Lei, Y.; Li, X.; Huang, J.; Liu, J. Direct Z-Scheme Heterojunction Catalysts Constructed by Graphitic-C3N4 and Photosensitive Metal-Organic Cages for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 890. https://doi.org/10.3390/nano12050890
Lv C, Qin S, Lei Y, Li X, Huang J, Liu J. Direct Z-Scheme Heterojunction Catalysts Constructed by Graphitic-C3N4 and Photosensitive Metal-Organic Cages for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials. 2022; 12(5):890. https://doi.org/10.3390/nano12050890
Chicago/Turabian StyleLv, Chuying, Su Qin, Yang Lei, Xinao Li, Jianfeng Huang, and Junmin Liu. 2022. "Direct Z-Scheme Heterojunction Catalysts Constructed by Graphitic-C3N4 and Photosensitive Metal-Organic Cages for Efficient Photocatalytic Hydrogen Evolution" Nanomaterials 12, no. 5: 890. https://doi.org/10.3390/nano12050890